Antibiotic and hormone combined hydrogel capable of being locally injected as well as preparation method and application
A technology for local injection and antibiotics, which is applied in the direction of local antibacterial agents, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of short action time, fluid loss, and repeated puncture injections, etc., to achieve Good biocompatibility and uniform drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
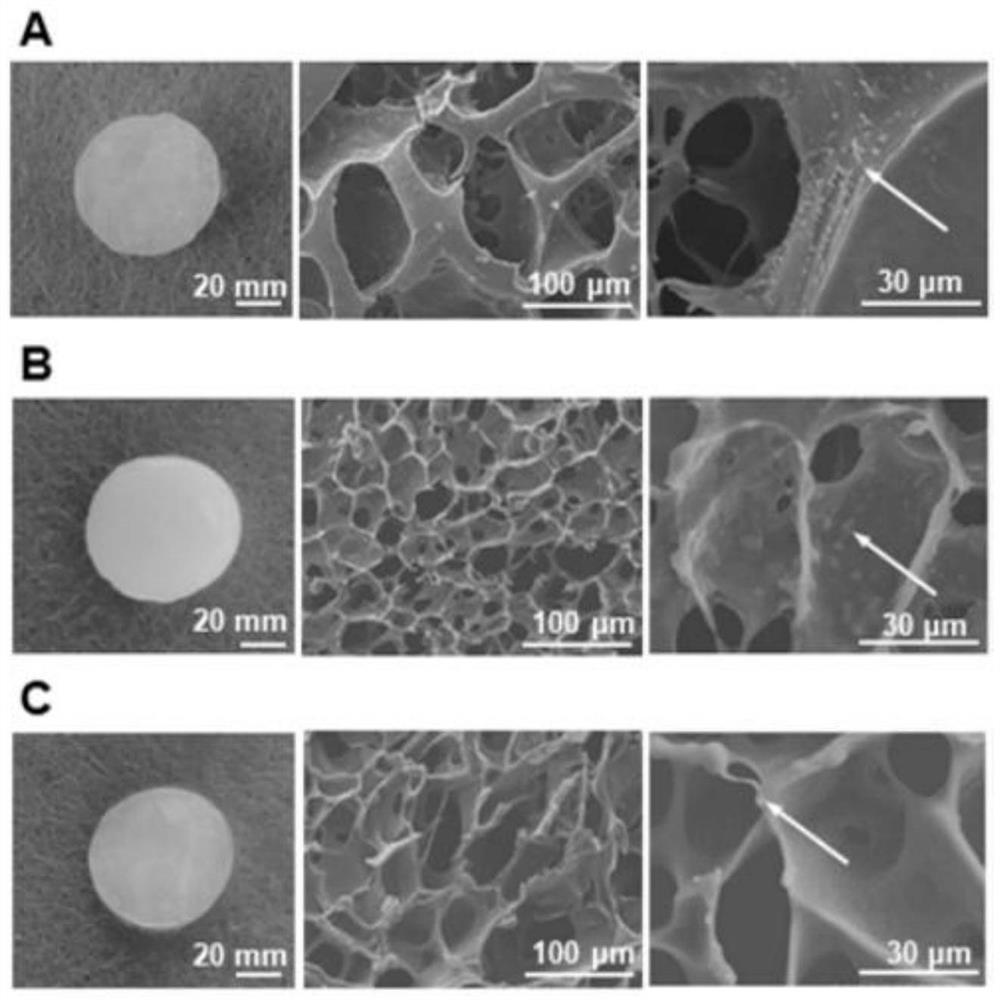
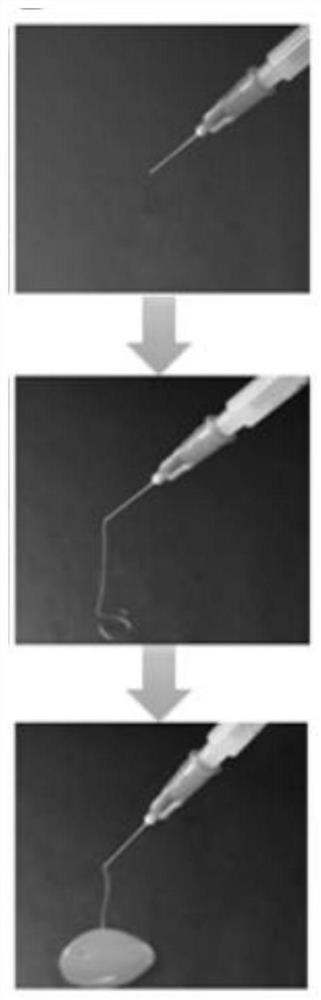
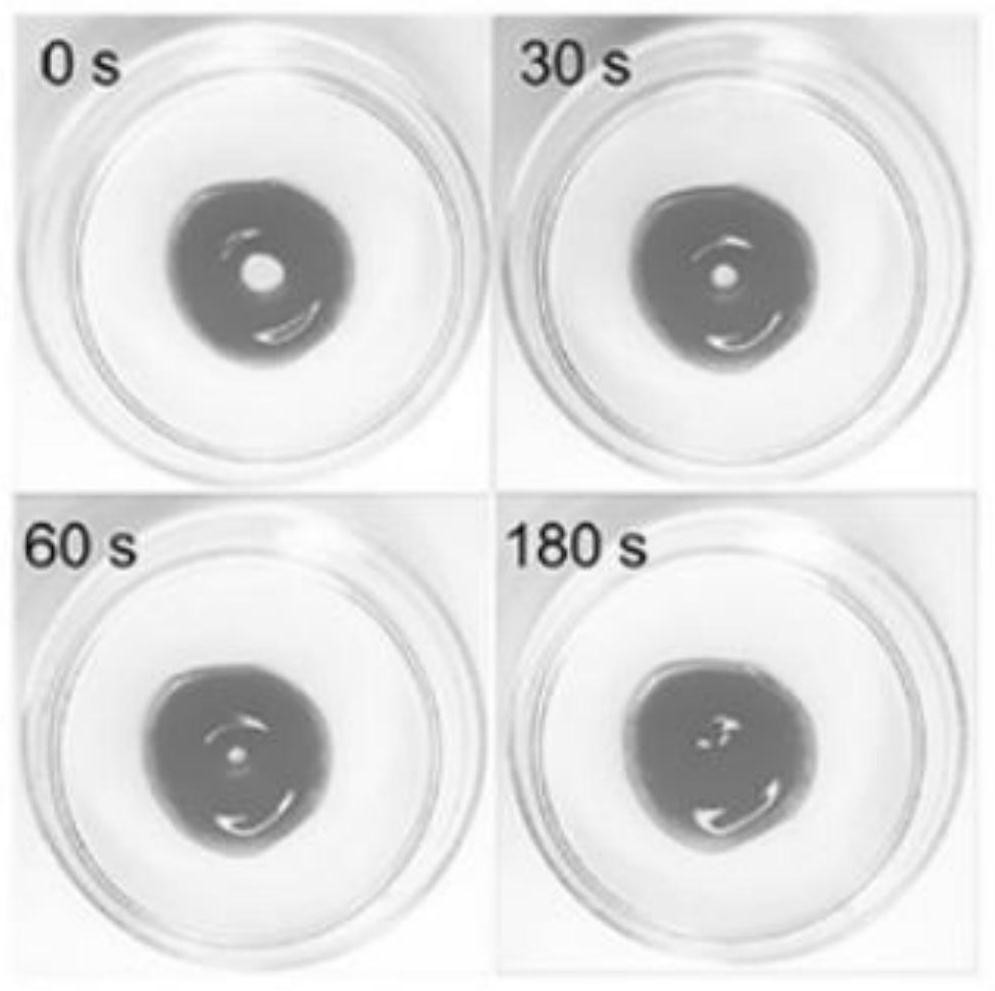
Image
Examples
Embodiment 1
[0062] Embodiment 1 provides an injectable clarithromycin hydrogel and its preparation method.
[0063] The preparation method of injectable clarithromycin hydrogel comprises the following steps:
[0064] Step 1, dissolving lecithin, cholesterol and clarithromycin (CAM) in methanol at a weight ratio of 4:1:0.2 by ultrasonic waves to obtain a first solution.
[0065] Step 2: transfer the first solution to a rotary evaporator, and perform rotary evaporation under the conditions of a rotating speed of 50 r / min and a temperature of 40° C. to obtain a honeycomb-shaped thin film at the bottom of the container.
[0066] Step 3, mixing the above-mentioned film with a preheated buffer solution with a concentration of 1×PBS, then hydrating for 2 hours at a rotation speed of 50 r / min and a temperature of 40° C., and then ultrasonicating to obtain clarithromycin liposomes, Denoted as CAM-Lips. Wherein, the thin film and the PBS buffer solution are mixed according to the mass volume rati...
Embodiment 2
[0069] Example 2 provides a locally injectable hydrogel combined with antibiotics and hormones and a preparation method thereof. Wherein, the antibiotic used in this embodiment is CAM, and the hormone is budesonide (BUD).
[0070] The preparation method of locally injectable antibiotic and hormone combined hydrogel comprises the following steps:
[0071] Step 1, dissolving lecithin, cholesterol, CAM and BUD in methanol at a weight ratio of 4:1:0.2:0.2 by ultrasonic waves to obtain a mixed solution.
[0072] Step 2: transfer the mixed solution to a rotary evaporator, and perform rotary evaporation under the conditions of a rotating speed of 50 r / min and a temperature of 40° C. to obtain a honeycomb-shaped thin film at the bottom of the container.
[0073] Step 3, mixing the above film with preheated 1×PBS buffer solution, then hydrating for 2 hours under the conditions of rotating speed of 50r / min and temperature of 40°C, and then ultrasonicating to obtain liposomes for combin...
Embodiment 3
[0076] Embodiment 2 provides a kind of locally injectable hydrogel used as a control, and its preparation method is as follows:
[0077] Dissolve 4-ARM-PEG powder in deionized water and add 0.05M AgNO 3 The solution is mixed to obtain a clear, injectable hydrogel, referred to as Pure Hydrogel. Wherein, the purity of 4-ARM-PEG powder is 96%, the molecular weight is 10kDa, and the mass volume ratio of 4-ARM-PEG powder to deionized water is 1 mg:10 μl. Deionized water and AgNO 3 The volume ratio of the solution is 1:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com