Preparation method of 6,7-epoxy-gamma-ionone
A technology of ionone and epoxy, applied in the field of preparation of 6,7-epoxy-γ-ionone, can solve the problems of long reaction route, low yield, unfriendly environment, etc., and achieve short reaction time and high production efficiency The effect of low cost and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
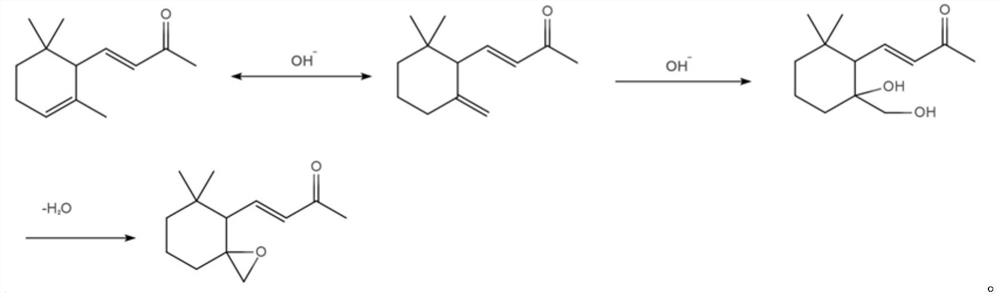
[0026] The object of the present invention is to provide a kind of preparation method of 6,7-epoxy-γ-ionone, comprising the following steps:
[0027] (1) reacting α-ionone and NaOH in a dioxane solvent to obtain a neutral reaction solution;
[0028] (2) post-processing the neutral reaction solution obtained in step (1) to obtain the crude product of 6,7-epoxy-γ-ionone;
[0029] (3) Purifying the crude 6,7-epoxy-γ-ionone obtained in step (2) to obtain pure 6,7-epoxy-γ-ionone.
[0030] In one embodiment of the present invention, in step (1), the NaOH is an aqueous NaOH solution with a mass percentage of 10-30%; preferably, the NaOH aqueous solution is 25% by mass.
[0031] In one embodiment of the present invention, in step (1), the feeding ratio of the α-ionone, NaOH aqueous solution and dioxane is 1mol: 1-4mol: 1-3L.
[0032] In one embodiment of the present invention, in step (1), the reaction temperature is 50° C., and the reaction time is 6 to 9 hours; after the reaction,...
Embodiment 1
[0048] This embodiment provides a preparation method of 6,7-epoxy-γ-ionone, comprising the following steps:
[0049] (1) At 50°C, a mixed solution consisting of 5.00 grams (99.80%, 25.99mmoL) of α-ionone, 10.40mL (25.99mmoL) and 25.99mL dioxane in 10% by mass percentage, After stirring and reacting for 6 hours, the obtained reaction solution was washed with 5% HCl aqueous solution by mass percentage, and the pH of the washing was neutralized;
[0050] (2) the step (1) pH washing to neutral reaction solution is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4Dry and filter with filter paper the next day, and the obtained filtrate is evaporated and concentrated by a rotary evaporator to obtain 4.01 g of crude product of 6,7-epoxy-γ-ionone, which is detected by gas chromatography. The purity is 93.70%, and the yield is 69.45% %;
[0051] (3) The crude product of 6,7-epoxy-γ-ionone obtained in step (2) is separated with a silica gel column, a...
Embodiment 2
[0055] This embodiment provides a preparation method of 6,7-epoxy-γ-ionone, comprising the following steps:
[0056] (1) At 50°C, a mixed solution composed of 5.29 grams of α-ionone (99.80%, 27.50mmol), 8.32mL (55.00mmol) of NaOH aqueous solution (55.00mmol) and 30mL of dioxane with a mass percent content of 25%, was stirred After reacting for 6 hours, the obtained reaction solution was washed with 10% HCl aqueous solution by mass percentage, and the pH of the washing was neutralized;
[0057] (2) step (1) pH washing to neutral reaction solution is extracted with ether, and the organic layer of gained is washed with anhydrous MgSO 4 Dry and filter with filter paper the next day, and the obtained filtrate is evaporated and concentrated by a rotary evaporator to obtain 4.37g of crude product of 6,7-epoxy-γ-ionone, which is detected by gas chromatography and has a purity of 95.65%, and a yield of 73.08% %;
[0058] (3) The crude product of 6,7-epoxy-γ-ionone obtained in step (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com