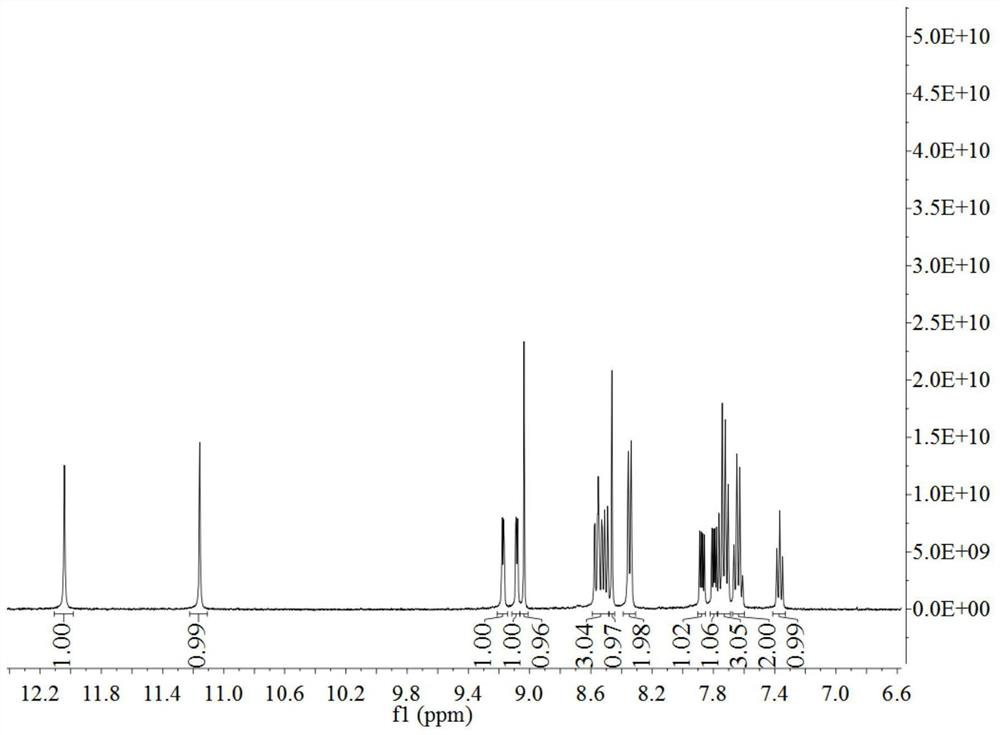
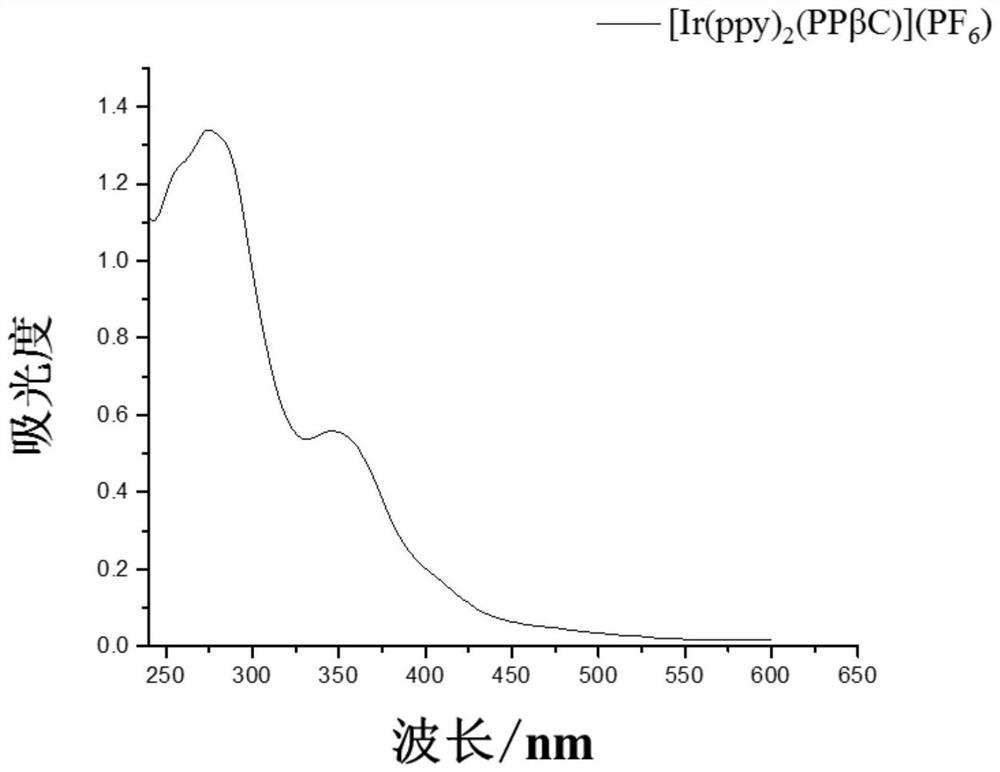
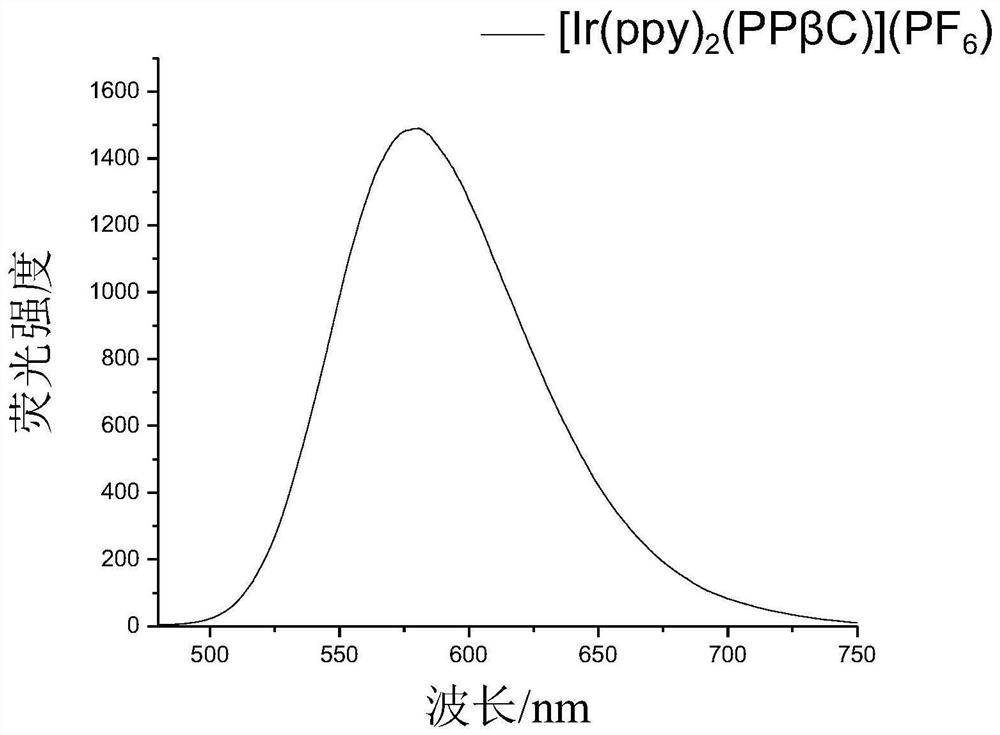
Beta-carboline derivative as well as preparation method and application thereof
A technology of derivatives and carbolines, which is applied in the field of β-carboline derivatives and its preparation, can solve the problems of reduced curative effect due to drug resistance, decreased therapeutic effect, and restrictions on long-term use, and achieves obvious anti-tumor effects, significant phototoxicity, and The effect of the novel mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0265] The present embodiment is a preparation method of β-carboline derivatives, comprising the following steps:
[0266] S1. Preparation of compound 2:
[0267]
[0268] Tryptophan (compound 1, 0.82g, 4mmol) was dissolved in 20mL of methanol, and thionyl chloride (0.29mL, 4mmol) was added dropwise under ice-cooling, condensed and refluxed at 100°C for 7h, and the obtained solution was distilled off under reduced pressure to remove the solvent. After rinsing with ethyl acetate, suction filtration and drying, 0.98 g of white powder (compound 2) was obtained with a yield of 96.4%.
[0269] S2, preparation of compound 3:
[0270]
[0271] Add compound 2 (1.019g, 4mmol) to the reaction tube, then add benzaldehyde (408μL, 4mmol), dissolve in 15mL of isopropanol, heat and reflux at 90°C for 10h under the protection of argon, and distill off the solvent from the obtained liquid under reduced pressure , add benzene to stir, filter with suction, and dry to obtain 1.12 g of lig...
Embodiment 2
[0287] This embodiment is a preparation method of an iridium complex, comprising the following steps:
[0288] S1. Preparation of Compound 8:
[0289]
[0290] IrCl 3 ·nH 2 O (1.192g, 2mmol) and ppy (0.62g, 4mmol) were added to a mixed solution of ethylene glycol-ether and water (3:1, v / v) at a molar ratio of 1:2, heated to reflux for 24h, filtered, The crude product was passed through a silica gel column to obtain 0.90g Ir 2 (ppy) 4 Cl 2 (Compound 8), yield 84.0%.
[0291] S2. Preparation of compound 9:
[0292]
[0293] Ir 2 (ppy) 4 Cl 2 (Compound 8, 2.054g, 2mmol) and PPβC (Compound 7, 1.86g, 4mmol) were dissolved in the mixed solution of dichloromethane / methanol (2:1, v / v), refluxed for 4h under the protection of argon, and the reaction After the solution was cooled to room temperature, CH was removed by rotary evaporation. 2 Cl 2 After transferring to a beaker, 20 mL of KPF was added to the solution 6 saturated aqueous solution. After cooling overnight...
Embodiment 3
[0299] This embodiment is a preparation method of an iridium complex, comprising the following steps:
[0300] S1. Preparation of compound 10:
[0301]
[0302] IrCl 3 ·nH 2 O (1.192g, 2mmol) and dfppy (0.76g, 4mmol) were added to a mixed solution of ethylene glycol-ether and water (3:1, v / v) at a molar ratio of 1:2, heated to reflux for 24h, and filtered 0.91g Ir 2 (dfppy) 4 Cl 2 (Compound 10), yield 75.0%.
[0303] S2. Preparation of compound 11:
[0304]
[0305] Ir 2 (dfppy) 4 Cl 2 (2.432g, 2mmol) and compound 7 (1.86g, 4mmol) were dissolved in a mixed solution of dichloromethane / methanol (2:1, v / v), refluxed for 4h under the protection of argon, and the solution was cooled after the reaction to room temperature, rotary evaporation to remove CH 2 Cl 2 After transferring to a beaker, 20 mL of KPF was added to the solution 6 saturated aqueous solution. After cooling overnight at 4°C in the refrigerator, it was collected by filtration, dried in vacuo, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com