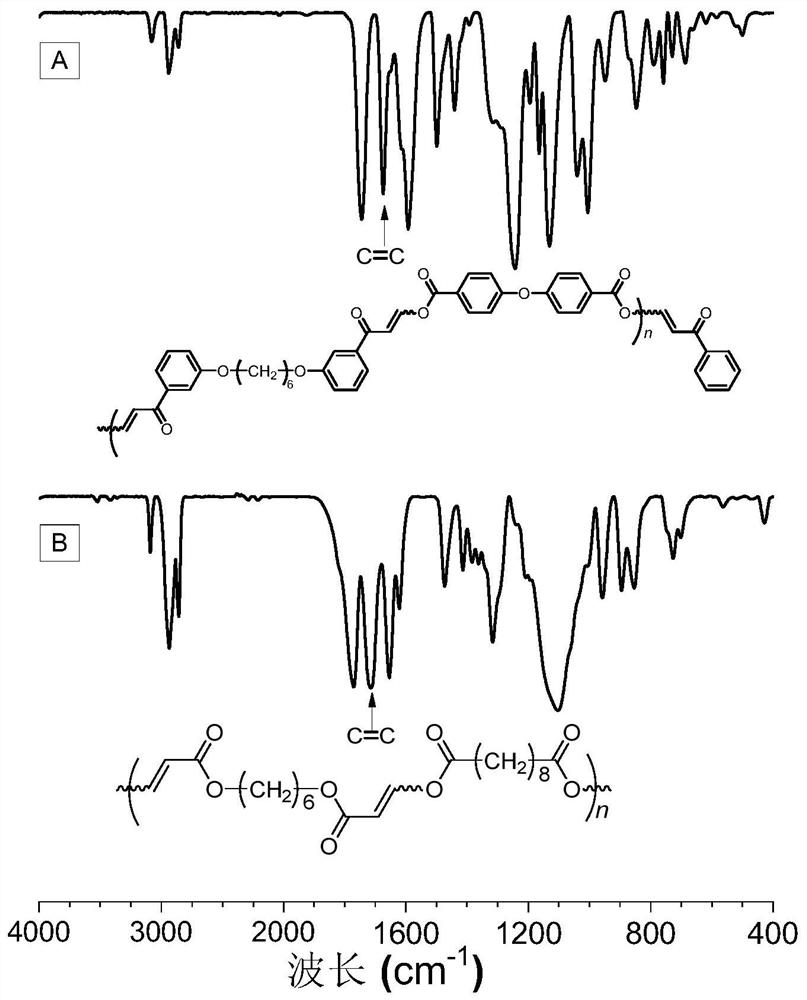
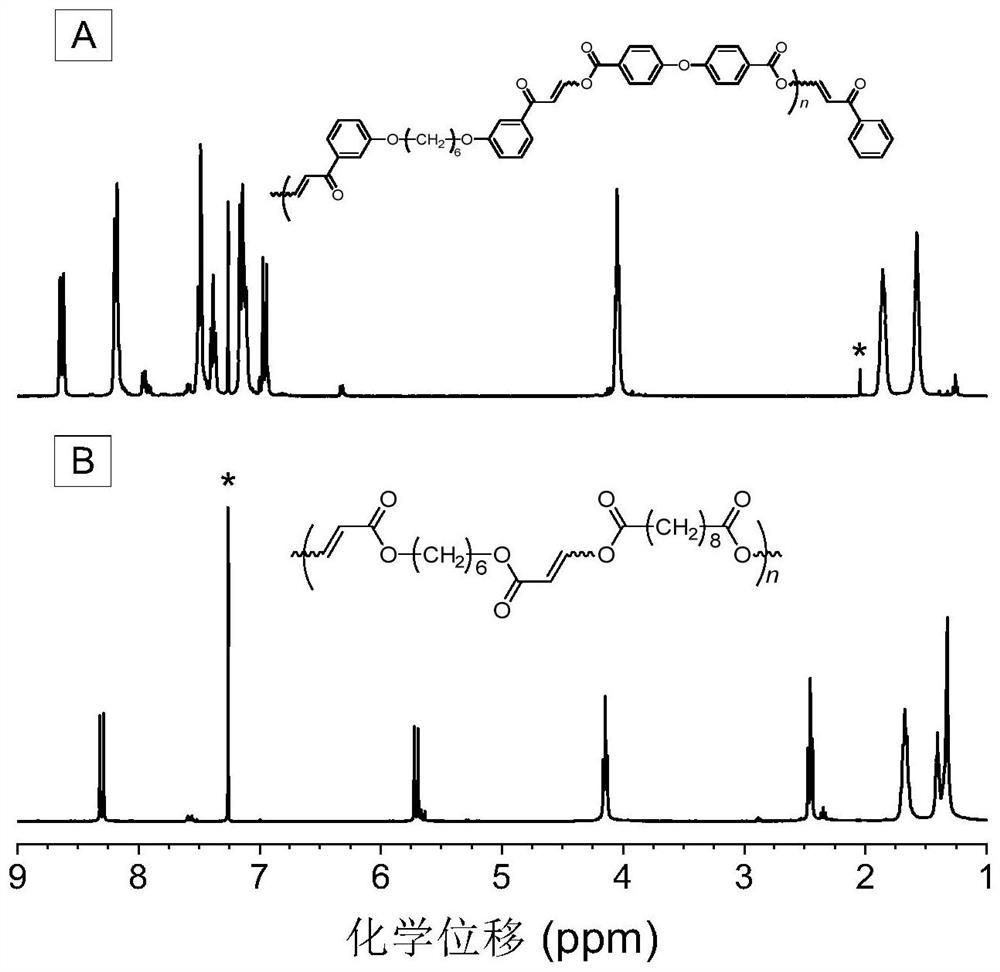
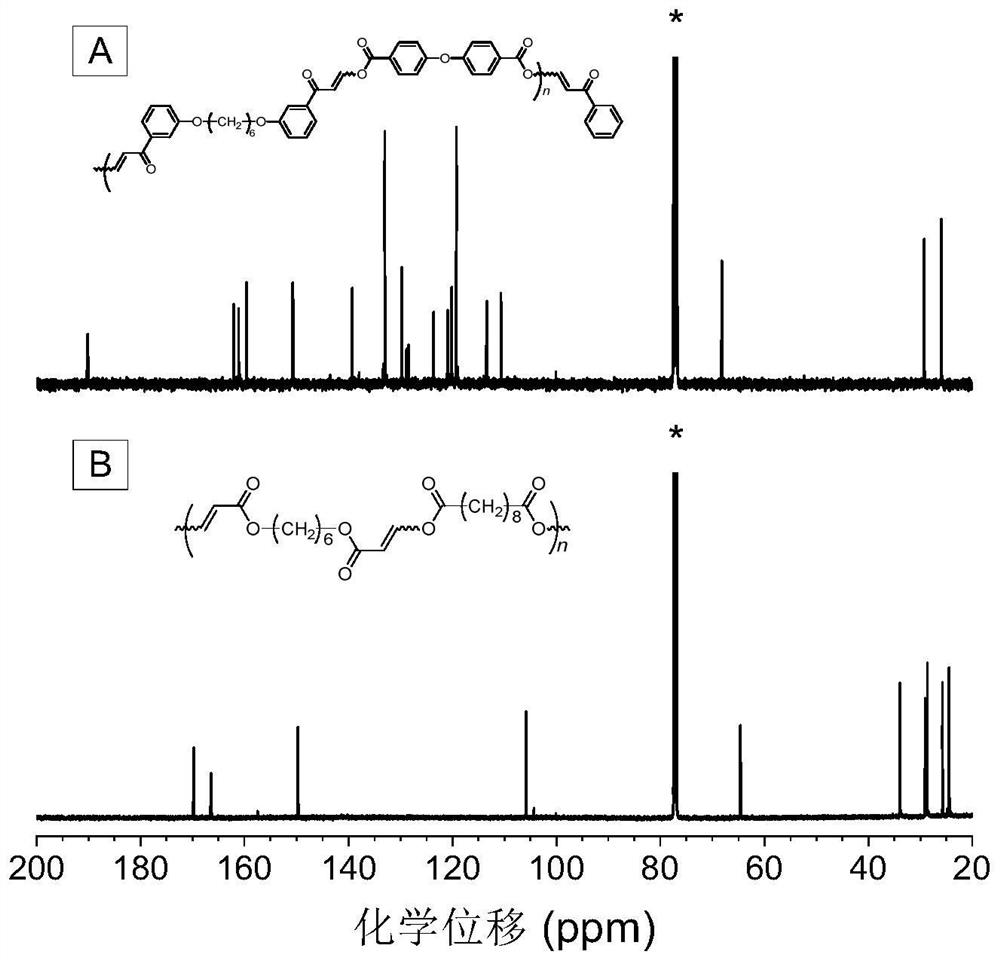
Method for preparing polycarbonyl enol ester compound by catalyzing alkyne carboxylic acid polymerization through organic base
An organic base catalyzed alkyne carboxylic acid and polycarbonyl enol ester technology, which is applied in the fields of polymer chemistry and materials science, can solve the problems of inability to achieve atom utilization, and achieve the effects of low price, mild reaction conditions and wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A polycarbonyl enol ester compound is prepared by polymerizing a dibasic carbonyl alkyne compound and a dibasic carboxylic acid compound, and the reaction equation is as formula (1):
[0054]
[0055] Among them, the synthesis method of monomer M1 can be synthesized according to the synthesis method of the applicant in the published literature (Macromolecules, 2020, 53, 2516-2525); M2 is 4,4'-diphenyl ether dicarboxylic acid, which can be purchased from the market In this example, it was purchased from Anaiji Chemical; the catalyst DABCO can be purchased from the market, and in this example and the examples described below, it was purchased from TCIA (Shanghai) Chemical Industry Development Co., Ltd.
[0056] The preparation steps of described polycarbonyl enol ester compound are as follows:
[0057] Weigh 37.4mg (0.1mmol) of monomer M1, 25.8mg (0.1mmol) of monomer M2 into a 10mL polymerization tube, and then add 0.9mL tetrahydrofuran / N,N-dimethylformamide (volume ra...
Embodiment 2
[0060] A polycarbonyl enol ester compound is prepared by polymerizing a dicarbonyl alkyne compound and a dibasic carboxylic acid compound, and the reaction equation is as formula (2):
[0061]
[0062] Wherein, the synthesis method of monomer M1 is the same as that in Example 1; M3 is 2,2-bis(4-carboxyphenyl)hexafluoropropane, which can be purchased from the market, and in this example, it was purchased from Anaiji Chemical.
[0063] The preparation steps of described polycarbonyl enol ester compound are as follows:
[0064] Weigh 37.4mg (0.1mmol) of monomer M1, add 39.2mg (0.1mmol) of monomer M3 into a 10mL polymerization tube, and then add 0.9mL tetrahydrofuran / N,N-dimethylformamide (volume ratio 7 / 3) After the monomer is completely dissolved, the temperature of the reaction system is controlled at 25°C. Dissolve 0.56mg (0.005mmol) DABCO in 0.1mL tetrahydrofuran / N,N-dimethylformamide (volume ratio 7 / 3) mixed solvent, add the DABCO solution to the above monomer solution, ...
Embodiment 3
[0067] A polycarbonyl enol ester compound is prepared by polymerizing a dibasic carbonyl alkyne compound and a dibasic carboxylic acid compound, and the reaction equation is as formula (3):
[0068]
[0069]
[0070] Wherein, the synthesis method of monomer M1 is the same as that in Example 1; M4 is 1,4-cyclohexanedicarboxylic acid, which can be purchased from the market, and in this example, it was purchased from Anaiji Chemical.
[0071] The preparation steps of described polycarbonyl enol ester compound are as follows:
[0072] Weigh 37.4mg (0.1mmol) of monomer M1, add 17.2mg (0.1mmol) of monomer M4 into a 10mL polymerization tube, and then add 0.9mL tetrahydrofuran / N,N-dimethylformamide (volume ratio 7 / 3) After the monomer is completely dissolved, the temperature of the reaction system is controlled at 25°C. Dissolve 0.56mg (0.005mmol) DABCO in 0.1mL tetrahydrofuran / N,N-dimethylformamide (volume ratio 7 / 3) mixed solvent, add the DABCO solution to the above monomer s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com