Recombinant protein of human echinococcosis antigen and application thereof
A recombinant protein and hydatid disease technology, applied in the field of immunomedicine, can solve problems such as difficulty in protein selection, achieve high specificity and sensitivity, facilitate large-scale screening of human echinococcosis, and improve specificity and sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
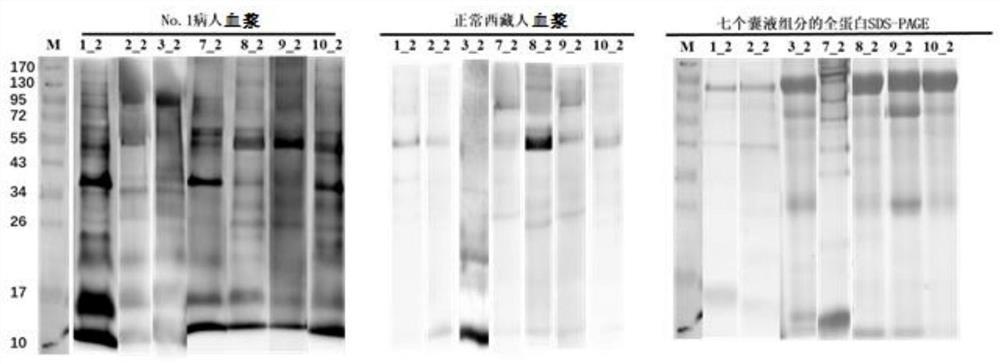
[0035] 1. Identification of hydatid protein from hydatid cysts isolated after operation of hydatid patients
[0036] 1) Firstly, the hydatid cysts isolated from 6 hydatid patients (the first batch) were divided into four parts according to their tissue structure to extract protein. From the inside to the outside, they are protoscoleum, cyst fluid, germinal layer and cuticle. ; According to whether the cyst fluid is transparent or not, the 6 hydatid cysts were divided into a transparent cyst fluid group and a non-transparent cyst fluid group.
[0037] 2) Protein extraction was performed on the four components of each hydatid cyst, and the extracted protein was subjected to liquid enzymatic hydrolysis, followed by LC-MS / MS identification using a QE mass spectrometer;
[0038] 3) The original data of the QE off-machine use maxquant protein identification software, use human and hydatid protein database collections, carry out hydatid protein identification, statistical identificat...
Embodiment 2
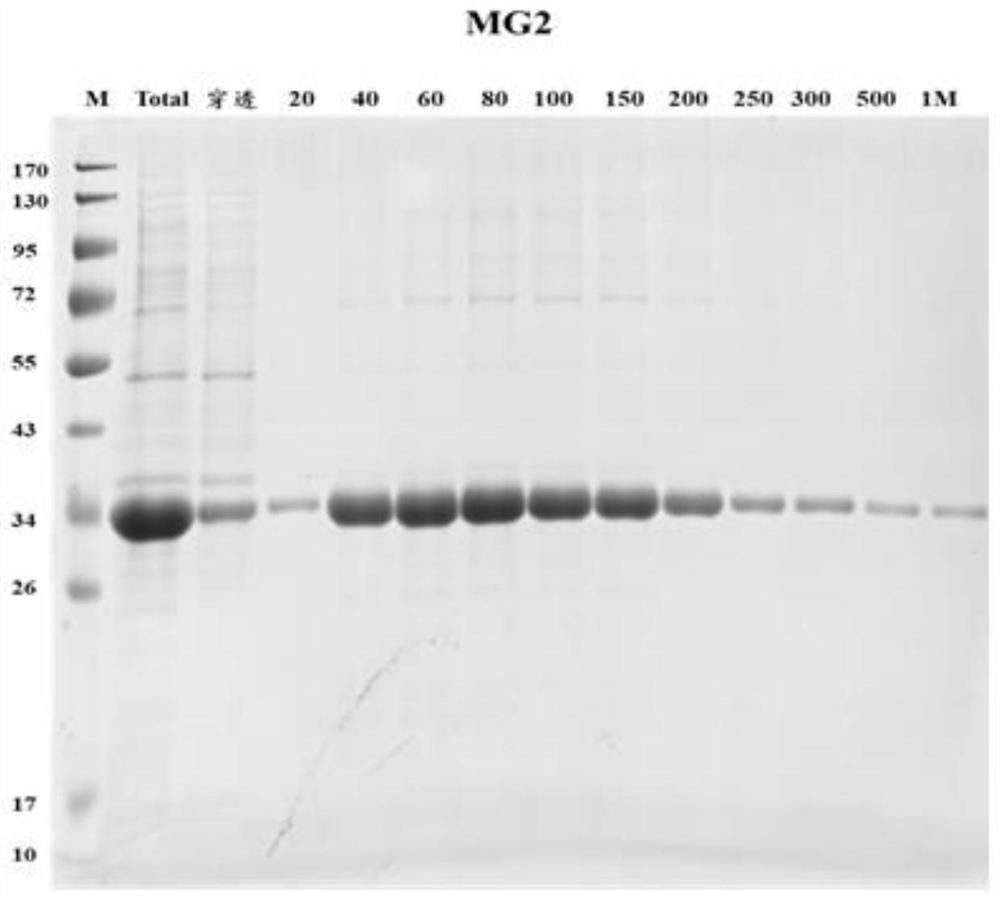
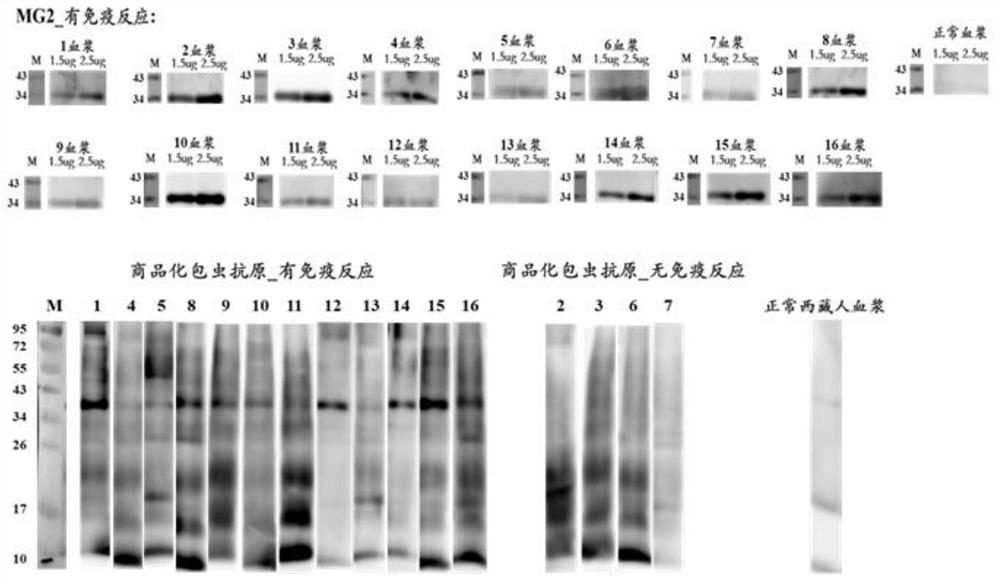
[0083] This embodiment uses the MG2 recombinant protein as described in Example 1, and the detection objects are 60 cases of B-ultrasound positive plasma (including suspected) samples and 33 cases of normal human plasma (determined as negative) samples, according to the standard operating procedures of indirect ELISA Implement validation.
[0084] 1. ELISA experimental operation steps
[0085] 1) Antigen quantification: Each antigen was blown evenly with a pipette gun before coating, and a micro-ultraviolet spectrophotometer was used to quantify the antigen to ensure that the protein was not degraded. If there is no absorption peak at A280, it means that the amount of antigen is very low and it is not suitable for coating; if the protein is precipitated or insoluble, add 8M urea dropwise and blow evenly, centrifuge to get the supernatant and use the Bradford method to re-quantify the protein concentration.
[0086] 2) Coating: compare the concentration of the antigen tube wal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com