Method for synthesizing 4-amino-3, 6-dichloropicolinic acid through electrolytic dechlorination, product and application
A technology of clopicolinic acid and triclopyralid, which is applied in the field of electrochemical synthesis, can solve the problems of "unstable electrolysis voltage, large amount of waste salt produced, high tank pressure in the later stage, etc., so as to avoid large alkali consumption, excellent quality, The effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
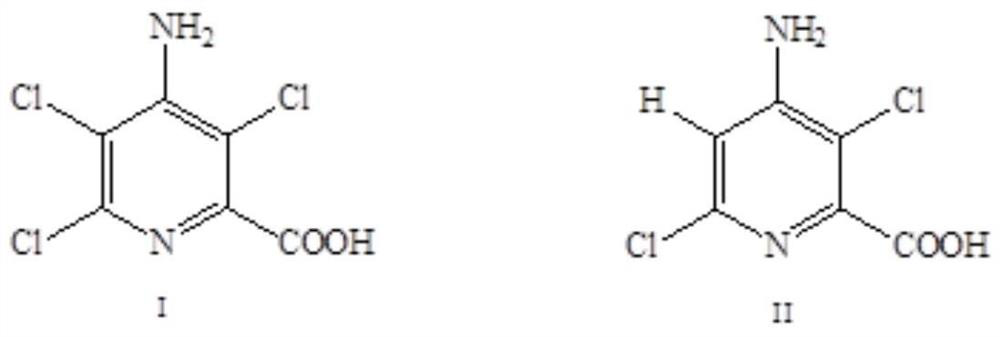
[0053] The method for synthesizing 4-amino-3,6-dichloropicolinic acid by electrolytic dechlorination of acidic anolyte:
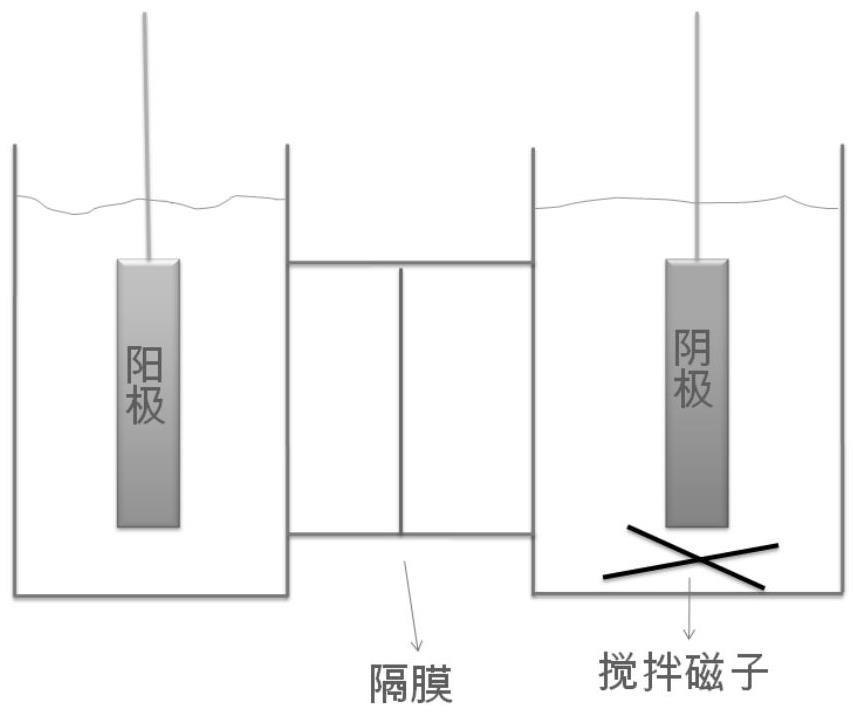
[0054] The electrolysis conditions are: in an H-type electrolytic cell with Nafion 324 cationic membrane as a diaphragm (such as figure 1 ); the activated silver mesh electrode prepared by the above method is the cathode, and the titanium-based iridium oxide coating sheet (geometric size is 0.5cm × 4.0cm × 6.0cm) of the same area is the anode, and the distance between the anode and cathode is 6cm; 200mL Contains 0.5M H 2 SO 4 The aqueous solution is the anolyte, and the 100mL aqueous solution containing 0.3M 4-amino-3,5,6-trichloropicolinic acid + 0.6M NaOH is the catholyte. Stir catholyte at 30-35°C and feed 2A / dm 2 The electric current, stop electrolysis after reacting for 6 hours.
[0055] During the electrolysis, it was observed that the voltage between the cathode and anode stabilized in the range of 4.4–5.1 V.
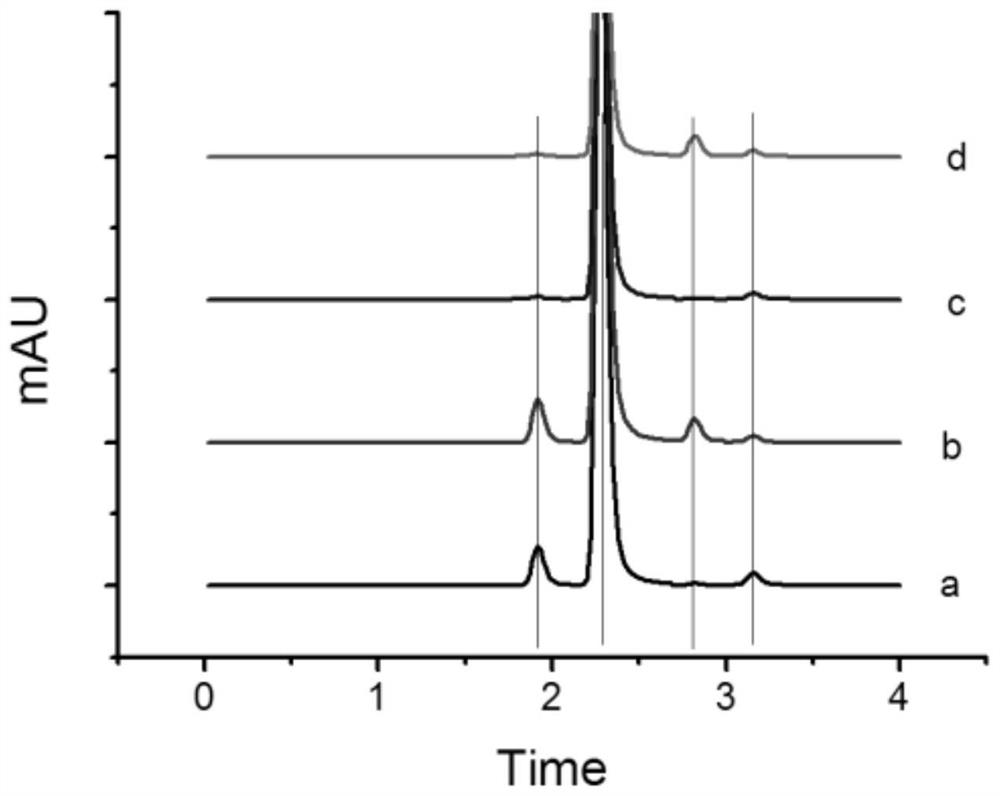
[0056] After the electrolysis end...
Embodiment 2
[0059] The method for synthesizing 4-amino-3,6-dichloropicolinic acid by electrolytic dechlorination of acidic anolyte:
[0060] The electrolysis conditions are: in an H-type electrolytic cell with Nafion 324 cationic membrane as a diaphragm (such as figure 1 ); the activated silver mesh electrode prepared by the above method is the cathode, and the titanium-based iridium oxide coating sheet (geometric size is 0.5cm × 4.0cm × 6.0cm) of the same area is the anode, and the distance between the anode and cathode is 6cm; 200mL Contains 2.0M H 2 SO 4 The aqueous solution is the anolyte, and the 100mL aqueous solution containing 0.6M 4-amino-3,5,6-trichloropicolinic acid + 1.2M NaOH is the catholyte. Stir catholyte at 55-60°C and feed 5A / dm 2 After 2 hours of reaction, adjust the current density to 2A / dm 2 Electrolysis was performed for another 5 hours, and the electrolysis was stopped.
[0061] During electrolysis, it was observed that the voltage between the cathode and anod...
Embodiment 3
[0065] The method for synthesizing 4-amino-3,6-dichloropicolinic acid by electrolytic dechlorination of acidic anolyte:
[0066] The electrolysis conditions are: in an H-type electrolytic cell with Nafion 324 cationic membrane as a diaphragm (such as figure 1 ); the activated silver mesh electrode prepared by the above method is the cathode, and the titanium-based iridium oxide coating sheet (geometric size is 0.5cm × 4.0cm × 6.0cm) of the same area is the anode, and the distance between the anode and cathode is 6cm; 200mL Contains 5.0M H 2 SO 4 The aqueous solution is the anolyte, 100mL contains 1.0M 4-amino-3,5,6-trichloropicolinic acid + 1.0M K 2 CO 3 The aqueous solution is catholyte (pH ~ 8). At 85-90°C, stir the catholyte and feed it with 10A / dm 2 The electric current, stop electrolysis after reacting for 5 hours.
[0067] During electrolysis, it was observed that the voltage between the cathode and anode stabilized in the range of 4.5–4.7 V.
[0068] After the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com