Catalyst for deep removal of CO, and preparation method thereof
A catalyst and removal technology, used in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of not being able to drop below 2ppm, and achieve low equipment requirements, mild reaction conditions, and preparation methods. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
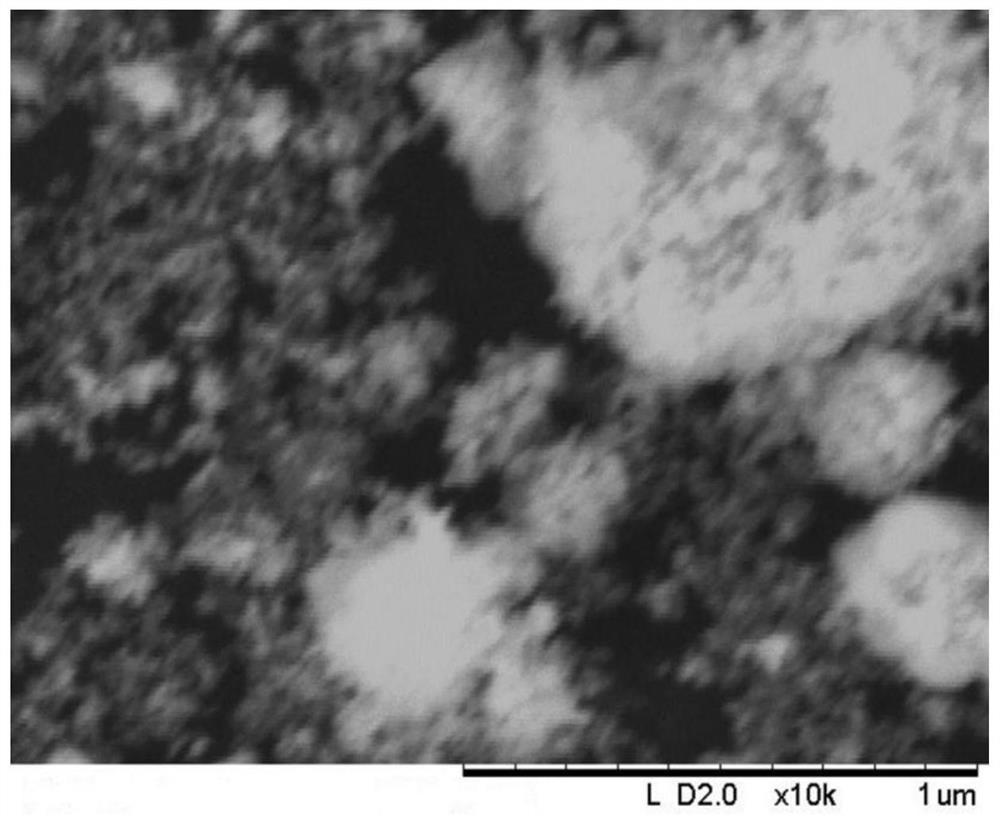
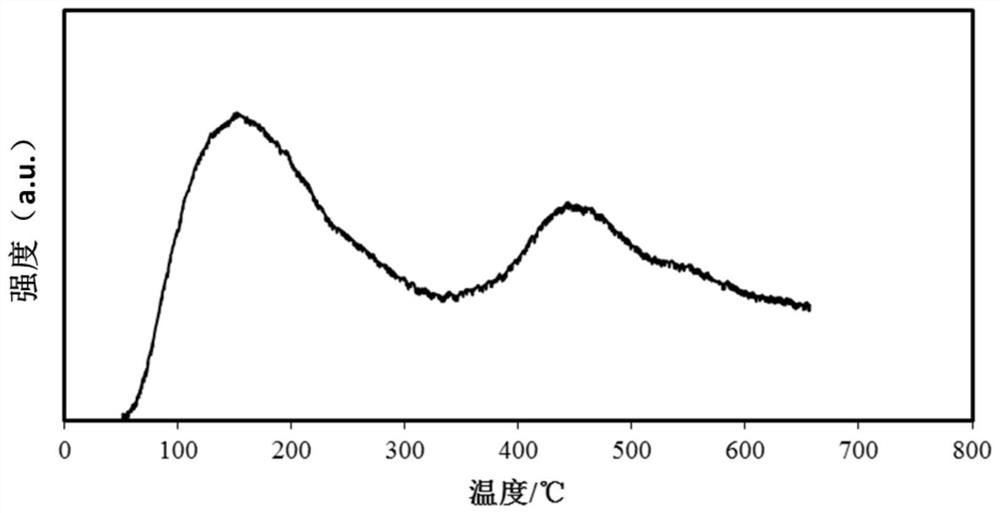
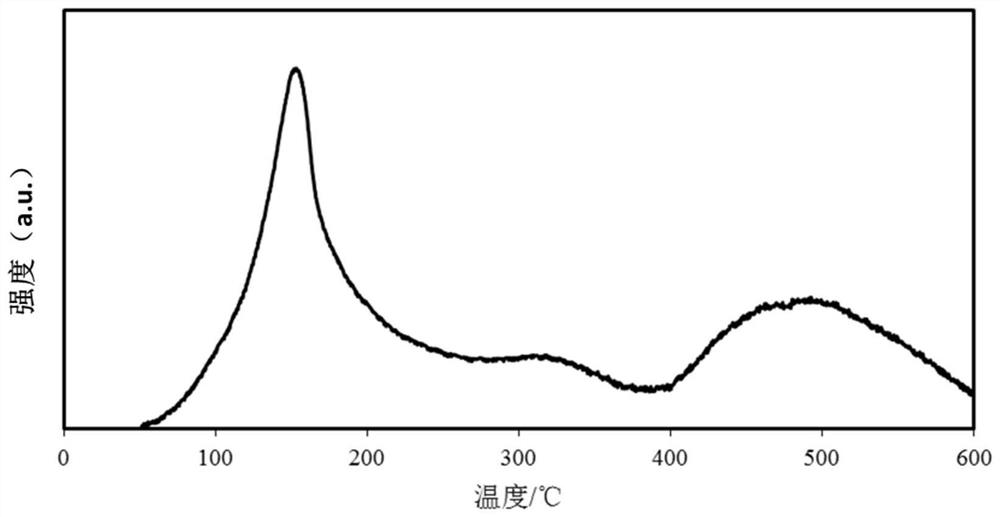
Image
Examples
Embodiment 1
[0038] (1) 25.8 g of a five-nitrate zirconium is dissolved in 120 ml of deionized water, placed in a reactor with a polytetrafluoroethylene liner, adding 7.2 g of urea, reacting at 120 ° C for 4 h, resulting in a hydrothermal reaction product Zr (OH) 4 Colloid, filtered the reaction product, dried at 110 ° C overnight, 450 ° C in the muffle, 2 h under air atmosphere, to obtain Zro 2 powder.
[0039] (2) 11.6 g of nickel nickel nickel is made into solution, and the 5G Zro obtained by adding steps (1) 2 Powder, transferred to a three flask, stir, 7.2 g of urea, water bath heating to 90 ° C, maintained for 6 h, so that the urea is hydrolyzed, after washing, placed in an oven, and finally in Maver 450 ° C in the furnace, calcined under the air atmosphere, and the catalyst obtained by obtaining the depth removal CO was prepared.
[0040] The catalyst for the depth removal of CO produced in this embodiment is Zro 2 The carrier load NIO catalyst, the data obtained by testing the catalyst...
Embodiment 2
[0045] (1) 25.8 g of a five-nitrate zirconium is dissolved in 120 ml deionized water, placed in a reactor with a polytetrafluoroethylene liner, adding 21.6 g of urea, reacted at 120 ° C for 4 hours to obtain a hydrothermal reaction product. After the reaction product was filtered, after 110 ° C drying overnight, 550 ° C in the muffle, calcined under the air atmosphere for 4 h, resulting in Zro 2 powder.
[0046] (2) 5.82 g of nickel nickel nickel in 80 ml of nickel is formulated into a solution, and the 23.4 Gzro obtained by adding step (1). 2 Powder, transferred to three flasks, stir, add 3.0 g of urea, water bath heating to 75 ° C, maintaining the urea to hydrolyzate, the hydrothermal reaction product is filtered through the oven, and finally in Mao 450 ° C in the furnace, calcined under the air atmosphere, and the catalyst obtained from the depth removal CO was prepared.
[0047] The catalyst for the depth removal of CO produced in this embodiment is Zro 2 Carrier load NIO cata...
Embodiment 3
[0050] (1) 25.8 g of zirconium nitrate is dissolved in 120 ml of deionized water, add 10g Al 2 O 3 , Transferred to a three flask, stir, then add 12.6 g of urea, heating to 90 ° C, maintains 4 to 8 h, so that the urea is sufficiently hydrolyzed, and the obtained hydrothermal reaction product is filtered, 110 ° C drying overnight, after the horse 450 ° C in the furnace, calcined under the air atmosphere, get ZRO 2 -Al 2 O 3 powder.
[0051] (2) 5.82 g of nickel nickel nickel nickel into solution, add 7.39 g of step (1) to produce ZRO 2 -Al 2 O 3 Powder, transferred to a three flask, stir, add 3.6 g of urea, water bath to 90 ° C, maintained for 4 to 8 h, so that the urea is sufficiently hydrolyzed, after filtration of the hydrothermal reaction, placed in an oven drying overnight, finally A catalyst of the depth removal CO was prepared in a muffle furnace.
[0052] The catalyst for the depth removal of CO produced in this embodiment is Zro 2 -Al 2 O 3 Carrier load NIO catalyst, HO in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com