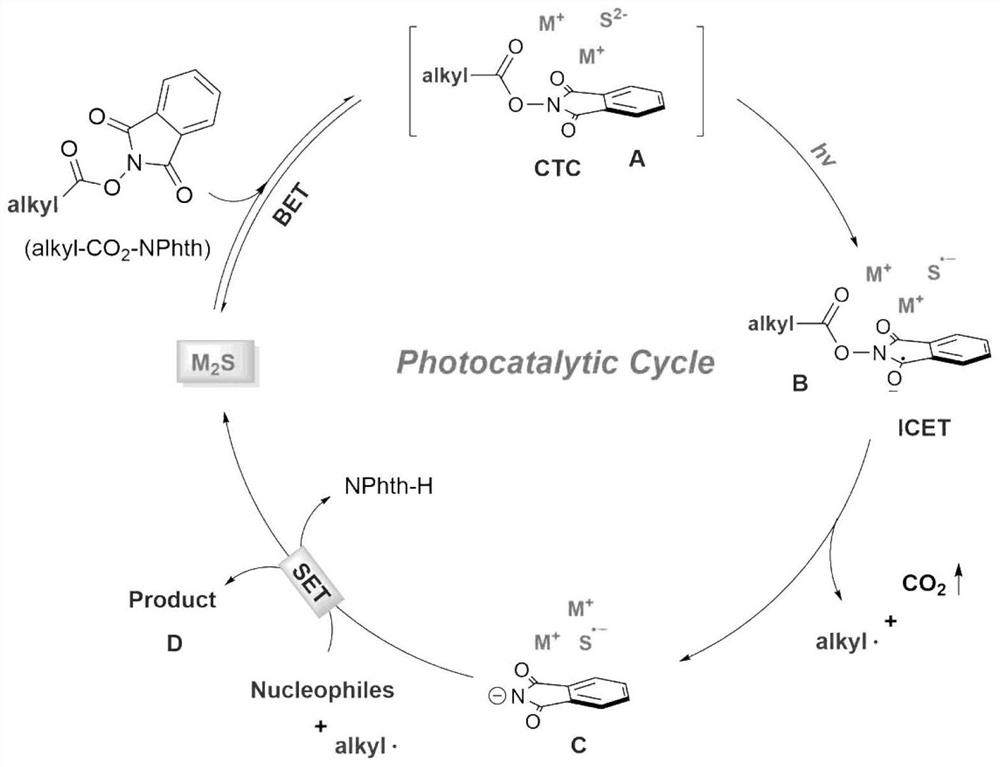
Method for photo-induced decarboxylation alkylation of alkyl-active carboxylic ester
A technology of alkyl active carboxylic acid, ester decarboxylation, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of complex synthesis, limited use, expensive photosensitizers, etc. Wide range of compounds and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Reaction formula:
[0080]
[0081] The specific method is as follows: in a 10mL Schlenk reaction tube (Beijing Xinweier Glass Instrument Co., Ltd., F891410 reaction tube, capacity 10mL, grinding port 14 / 20), add redox alkyl active carboxylate (0.3mmol) and lithium sulfide (0.04 mmol). The air in the tube was completely replaced with argon three times, and then 2 mL of N,N-dimethylacetamide (DMA) and free radical acceptor isocyanide (0.2 mmol) were added under an argon atmosphere. The reaction system was continuously stirred at room temperature for 12 hours under the irradiation of a 40W purple LED (427nm) lamp (IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). After the reaction was completed, the reaction was quenched with saturated NaCl solution, and the reaction solution was repeatedly extracted 3 times with 10 mL of ethyl acetate, and then the combined organic phase was concentrated by rotary evaporation (Swiss Buchi Co., Ltd., BUCHI rotary evapora...
Embodiment 2
[0088] Reaction formula:
[0089]
[0090] The method of embodiment 2 is the same as that of embodiment 1, the difference lies in the kind of redox alkyl active carboxylate shown in the above formula, and the productive rate of embodiment 2 is shown in table 1.
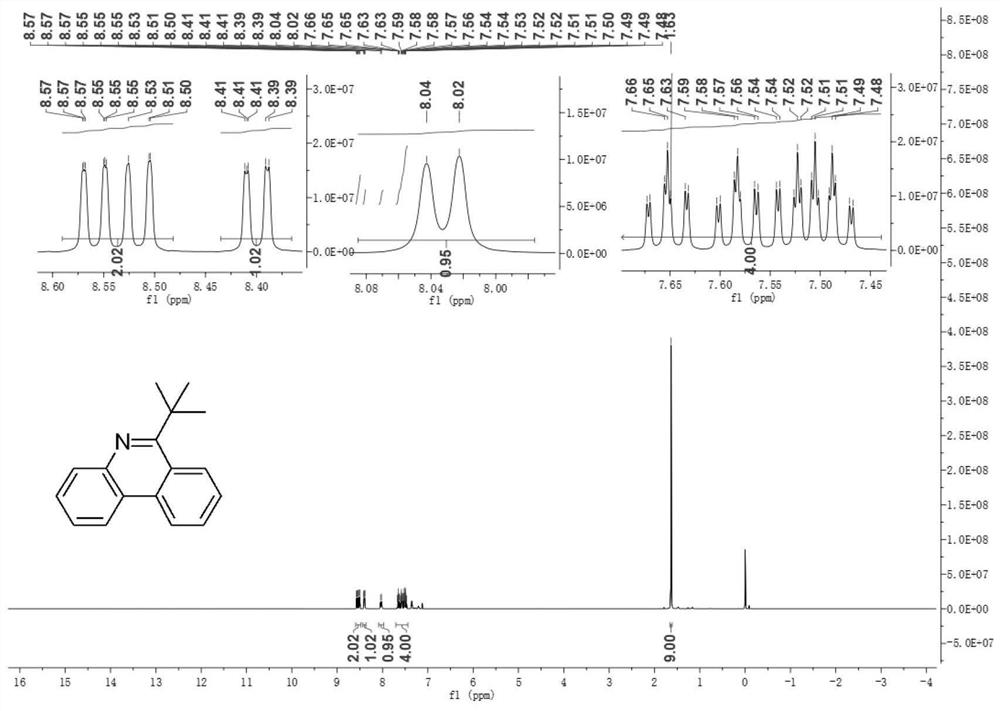
[0091] Utilize nuclear magnetic resonance to carry out the product of embodiment 2 gained 1 H NMR and 13 C NMR analysis.
[0092] Figure 4 It is the product in Example 2 of the present disclosure 1 H NMR nuclear magnetic resonance spectrum.
[0093] Such as Figure 4 as shown, 1 H NMR (400MHz, CDCl3) δ8.52(d, J=8.2Hz, 1H), 8.41(dd, J=8.3, 1.3Hz, 1H), 8.20(d, J=8.2Hz, 1H), 8.05(d ,J=8.2Hz,1H),7.59-7.57(m,4H),3.77-3.28(m,1H),2.01-1.72(m,7H),1.55-1.29(m,3H).
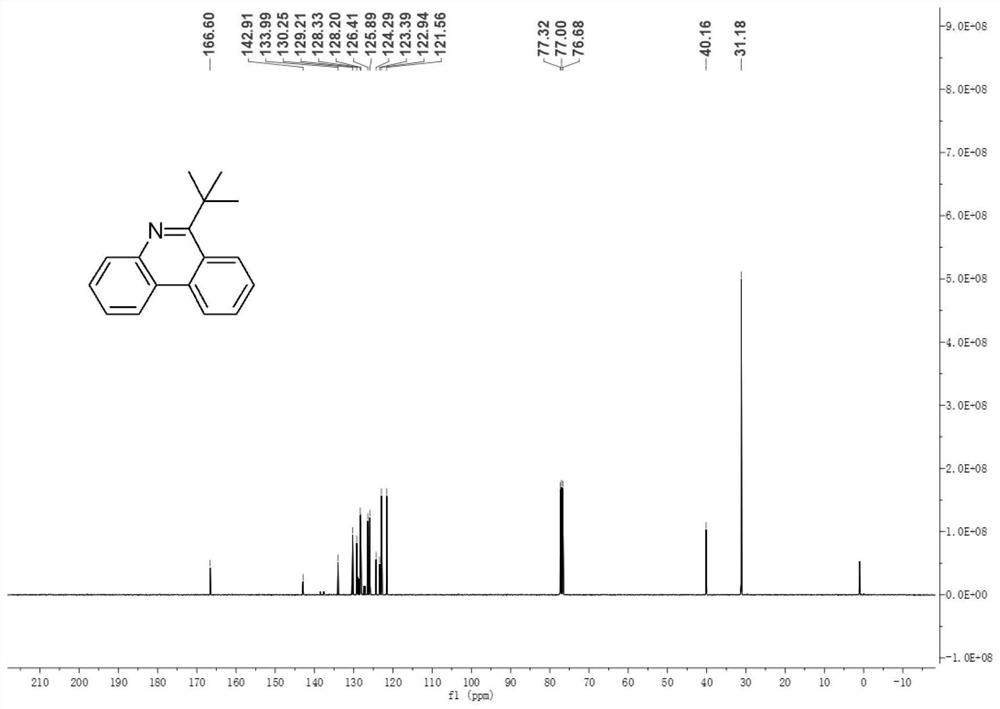
[0094] Figure 5 It is the product in Example 2 of the present disclosure 13 C NMR spectrum.
[0095] Such as Figure 5 as shown, 13 C NMR (101MHz, CDCl 3 )δ165.2, 143.8, 132.9, 129.8, 128.3, 127.0, 126.0, 125.5, 124.6, 123.2, 122.5, 121.7, 41.9, ...
Embodiment 3
[0097] Reaction formula:
[0098]
[0099] The method of Example 3 is the same as that of Example 1, except for the type of free radical acceptor isocyanide shown in the above formula. The yield of Example 3 is shown in Table 1.
[0100] The product obtained in embodiment 3 is carried out nuclear magnetic resonance analysis, and the results are as follows:
[0101] 1 H NMR (400MHz, CDCl 3 )δ8.57(d, J=9.0Hz, 1H), 8.41(dd, J=8.1, 1.5Hz, 1H), 8.21-8.03(m, 1H), 7.98(d, J=2.6Hz, 1H), 7.66-7.53(m,2H),7.41(dd,J=9.1,2.5Hz,1H),3.97(s,3H),1.73(s,9H).
[0102] 13 C NMR (101MHz, CDCl 3 )δ165.7, 157.2, 130.1, 128.2, 127.4, 126.5, 125.4, 124.4, 123.5, 121.1, 119.1, 114.0, 109.7, 55.4, 40.0, 30.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com