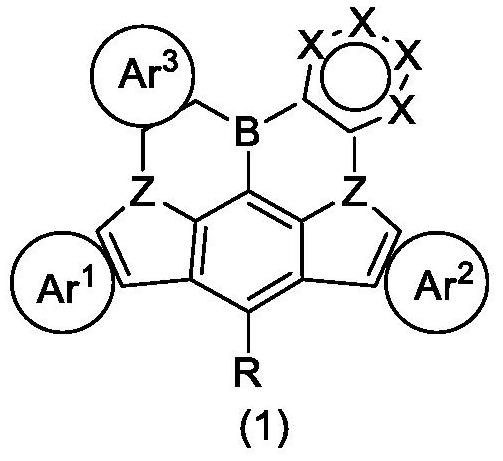
Boron-containing heterocyclic organic compound, mixture, composition, and organic electronic device
A technology of organic compounds and boron heterocycles, applied in the field of electroluminescent materials, can solve problems such as performance gaps, and achieve the effect of improving luminous efficiency and lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] The synthetic route of compound (1) is as follows:
[0153]
[0154] (1) Synthesis of Intermediate 1-3:
[0155] Under nitrogen protection atmosphere, in a dry three-necked flask, add 10mmol intermediate 1-1 and 10mmol intermediate 1-2, 0.2mmol palladium acetate, 1.38 grams of potassium carbonate respectively, add 150mL of tetrahydrofuran to dissolve it, and heat to 80 ℃ to the reaction liquid reflux, react for 12 hours, after the reaction is complete, add water to extract the reaction, and at the same time extract the organic phase with dichloromethane, combine and wash the organic phase several times, dry with anhydrous magnesium sulfate, filter, and rotary evaporate to dryness to obtain Coarse, purified by flash column chromatography to obtain 5.74 mmol of intermediate 1-3, yield: 57.4%. MS (ASAP) = 434.2.
[0156] (2) Synthesis of Intermediate 1-5:
[0157] Under a nitrogen atmosphere, add 1 mmol of intermediate 1-3 and 1 mmol of intermediate 1-4 to a dry thre...
Embodiment 2
[0161] The synthetic route of compound (2) is as follows:
[0162]
[0163] (1) Synthesis of intermediate 2-3:
[0164] Under nitrogen protection atmosphere, in a dry three-necked flask, add 10mmol intermediate 2-1 and 10mmol intermediate 2-2, 0.2mmol palladium acetate, 1.38 g potassium carbonate respectively, add 150mL tetrahydrofuran to dissolve it, and heat to 80°C until the reaction solution was refluxed, and reacted for 12 hours. After the reaction was complete, the reaction was extracted with water, and the organic phase was extracted several times with dichloromethane. , and purified by flash column chromatography to obtain intermediate 2-3 with a molar weight of 6.93 mmol and a yield of 69.3%. MS (ASAP) = 924.1.
[0165] (2) Synthesis of intermediate 2-5:
[0166] Under a nitrogen atmosphere, add 1 mmol of intermediate 2-3 and 1 mmol of intermediate 2-4 to a dry three-necked flask, pour 100 mL of DMSO into a solvent, add dry K 2 CO 3 As a base, react at 120°C f...
Embodiment 3
[0170] The synthetic route of compound (3) is as follows:
[0171]
[0172] (1) Synthesis of intermediate 3-3:
[0173]Under nitrogen protection atmosphere, in a dry three-necked flask, add 10mmol intermediate 3-1 and 10mmol intermediate 3-2, 0.2mmol palladium acetate, 1.38 g potassium carbonate respectively, add 150mL tetrahydrofuran to dissolve it, and heat to 80°C until the reaction solution was refluxed, and reacted for 12 hours. After the reaction was complete, the reaction was extracted with water, and the organic phase was extracted several times with dichloromethane. , and purified by flash column chromatography to obtain intermediate 3-3 with a molar weight of 6.72 mmol and a yield of 67.2%. MS (ASAP) = 321.4.
[0174] (2) Synthesis of Intermediate 3-5:
[0175] Under a nitrogen atmosphere, add 1 mmol of intermediate 3-3 and 1 mmol of intermediate 3-4 to a dry three-necked flask, pour 100 mL of DMSO into a solvent, add dry K 2 CO 3 As a base, react at 120°C fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com