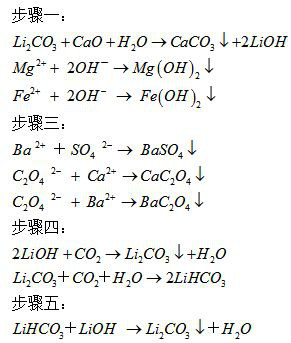Method for producing high-purity lithium carbonate by causticizing and carbonizing coarse lithium carbonate lime
A high-purity lithium carbonate and lithium carbonate technology, applied in lithium carbonate;/acid carbonate and other directions, can solve the problems of difficult to guarantee the content of raw materials impurities, high raw material cost, and high requirements for LiOH solution, and achieve quality assurance. , The effect of better quality control and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
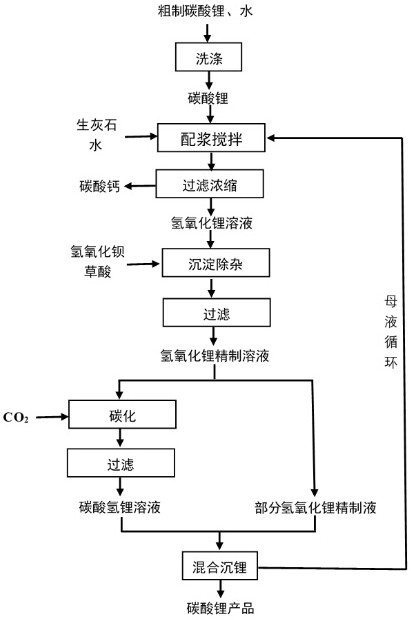
[0029] Example 1 Add crude lithium carbonate (containing 55wt% lithium carbonate) to the reaction tank, add water to wash and filter, then add quicklime and water, the liquid-solid ratio is 3, the molar ratio between quicklime and lithium carbonate is 1.1, and the stirring reaction temperature The temperature is 30°C, and the reaction time is 2 hours. Filter and separate the causticized calcium carbonate filter residue to obtain a lithium hydroxide solution (containing 13 g / L of lithium and 10 g / L of sulfate). Concentrate the lithium hydroxide solution to obtain lithium-containing 16 Lithium hydroxide solution in grams per liter, the added barium hydroxide and oxalic acid are stirred and reacted, the molar ratio of barium hydroxide and sulfate ion is 1:1.5, the amount of oxalic acid is 5 kg per cubic, and the refined solution of lithium hydroxide is obtained by filtration; Add half of the lithium hydroxide refined liquid into the carbonization reaction kettle and pass carbon di...
Embodiment 2
[0033]Add crude lithium carbonate (containing 75wt% lithium carbonate) to the reaction tank, add water to wash and filter, then add quicklime and water, the liquid-solid ratio is 3.5, the molar ratio between quicklime and lithium carbonate is 1.2, and the stirring reaction temperature is 60°C , the reaction time is 3 hours, filter and separate the causticized calcium carbonate filter residue and obtain a lithium hydroxide solution (containing 11 g / L of lithium and 10 g / L of sulfate), and concentrate the lithium hydroxide solution to obtain 17 g / L of lithium Lithium hydroxide solution, the added barium hydroxide and oxalic acid stirred and reacted, the molar ratio of barium hydroxide and sulfate ion was 1:1.25, the amount of oxalic acid was 6 kg per cubic meter, filtered to obtain a refined solution of lithium hydroxide; Lithium refined solution is added into the carbonization reaction kettle and carbon dioxide gas is passed into it for carbonization. The carbonization temperatu...
Embodiment 3
[0037] Add crude lithium carbonate (containing 55wt% lithium carbonate) into the reaction tank, add water to wash and filter, then add quicklime and water, the liquid-solid ratio is 3, the molar ratio between quicklime and lithium carbonate is 1.1, and the stirring reaction temperature is 30°C , the reaction time is 2 hours, filter and separate the causticized calcium carbonate filter residue and obtain a lithium hydroxide solution (containing lithium 10 g / L, sulfate radical 5 g / L), and concentrate the lithium hydroxide solution to obtain a lithium hydroxide solution containing 14 g / L Lithium hydroxide solution, the added barium hydroxide and oxalic acid stirred and reacted, the molar ratio of barium hydroxide and sulfate ion was 1:1.15, the amount of oxalic acid was 3 kg per cubic meter, filtered to obtain a refined solution of lithium hydroxide; Lithium refined solution is added into the carbonization reaction kettle and carbon dioxide gas is passed into it for carbonization....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com