Amino lipid as well as preparation method and application thereof
An amino lipid and alkyl technology, applied in the field of medicinal chemistry, can solve the problems of cytotoxicity and low efficiency, and achieve the effects of enhanced degradation ability, high atom economy, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Parallel synthesis and characterization of E7C71Ay series amino lipid compound library
[0066]
[0067] In the 25mL reaction tube, add FeCl 3 (4 mg, 0.005 mmol), Py (1 μL, 0.0025 mmol), 2-hexyldecanoic acid (0.3 mL, 1 mmol) and 1,2-epoxydodecane (0.27 mL, 1.2 mmol), stirred at room temperature overnight , to get Step I (1mmol), add 10mL of DCM, dubbed 0.1 M Step I solution.
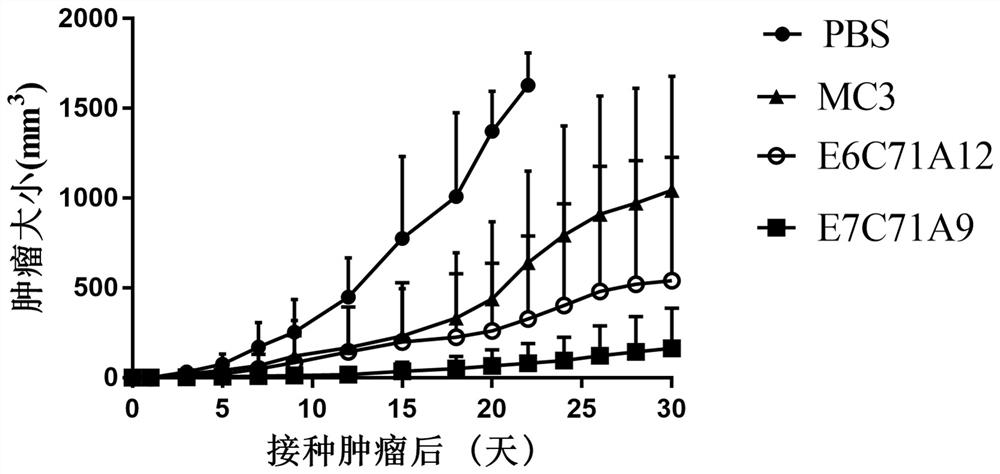
[0068] Transfer the Step I solution to 1.5 mL of 96-well plate with a pipette gun (0.1 mL each, 0.01 mmol), and add a DCM solution of carboxylic acid with tertiary amine (0.1 mL, 0.02 mmol, 0.2M), DIPEA and EDC·HCl in DCM (0.2 mL, 0.04 mmol, 0.2 M), DMAP in DCM (0.1 mL, 0.005 mmol, 0.05 M) were stirred at room temperature for 6 hours, and no Step I raw materials were detected by TLC. After the reaction was completed, it was waved to dryness at room temperature to obtain 15 amino lipid compounds E7C71Ay. Mass spectrometry was performed, and the results are shown in Table 1 below. ...
Embodiment 2
[0072] Example 2: 2-Hydroxyhexadecyldodecanoate
[0073]
[0074] In the 25mL reaction tube, add FeCl 3 (20mg, 0.025mmol), Py (5μL, 0.0125mmol), lauric acid (1g, 5mmol) and 1,2-epoxyhexadecane (1.7mL, 6mmol), stirred at room temperature overnight, using column Purification by analysis (hexane:EA=20:1 to 5:1) gave 2-hydroxyhexadecanyl dodecanoate (2.0 g, 90%). 1 H NMR (400MHz, CDCl 3 ): δ 0.88 (t, 6H), 1.26-1.45 (m, 40H), 1.47 (m, 2H), 1.63 (m, 2H), 2.02 (m, 1H), 2.34 (t, 2H), 3.82 (m , 1H), 3.95 (m, 1H), 4.13 (m, 1H). ESI-MS calculated for C 28 h 57 o 3 + [M+H] + 441.4, found 441.6.
Embodiment 3
[0075] Example 3: Hexadecyl 2-((4-(dimethylamino)butyryl)oxy)dodecanoate
[0076]
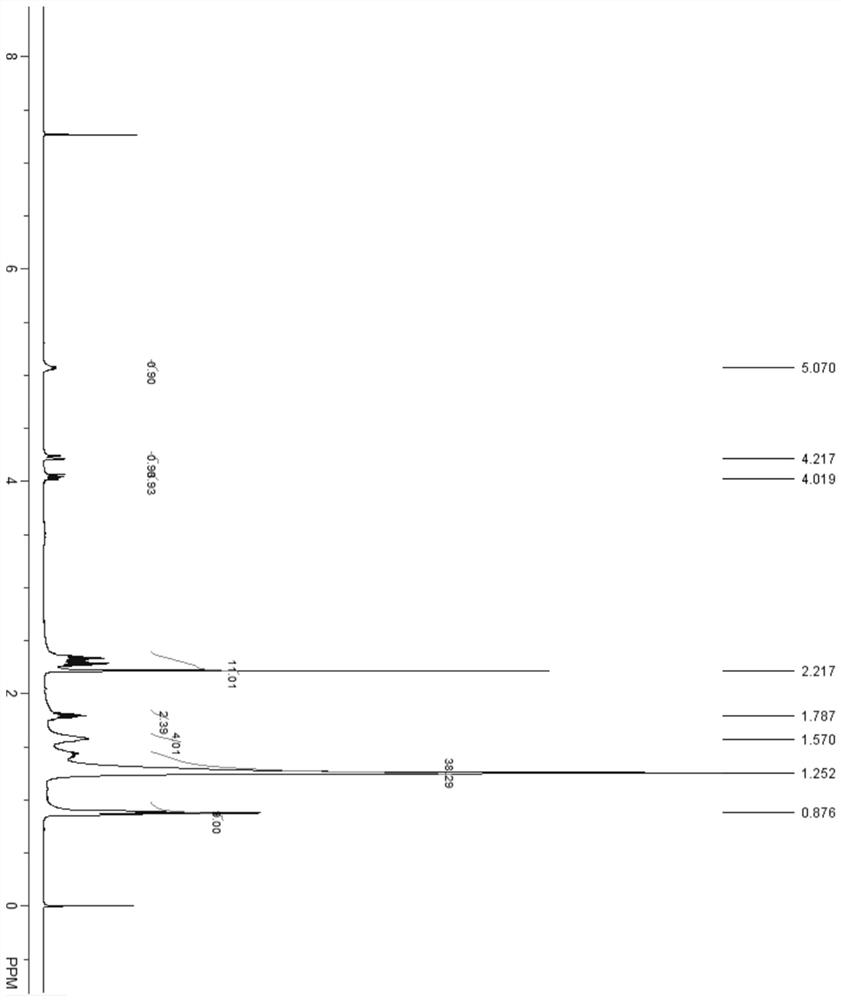
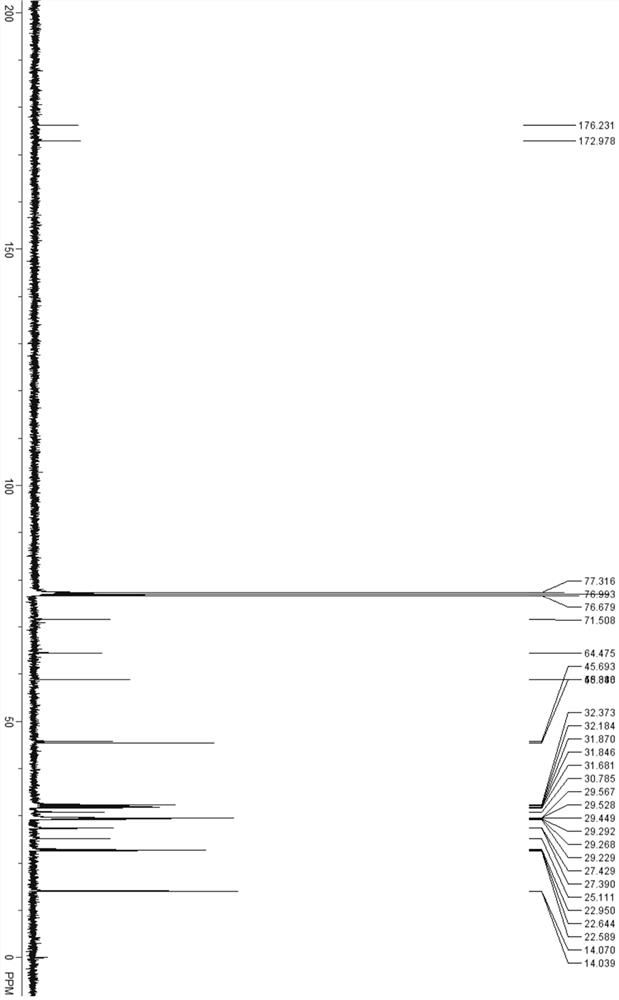
[0077]In a 10 mL reaction tube, add EDC·HCl (192 mg, 1 mmol), DIPEA (174 μL, 1 mmol), DMAP (3.0 mg, 0.025 mmol), 4-(dimethylamino)butyric acid (101 mg, 0.6 mmol) ), 2-hydroxyhexadecanoate (220 mg, 0.5 mmol), 4 mL DCM. Stirring at room temperature for 3 h gave compound E11C7A9 (235 mg, 85%). 1 HNMR (400 MHz, CDCl 3 ): δ 0.88 (t, 6H), 1.25-1.45 (m, 40H), 1.58 (m, 4H), 1.78 (m, 2H), 2.23 (s, 6H), 2.30 (m, 6H), 4.01 (m , 1H), 4.21 (m, 1H), 5.08 (m, 1H). 13 C NMR (400 MHz, CDCl 3 ): δ14.03, 14.08, 22.59, 22.64, 23.35, 25.11, 27.39, 27.43, 29.23, 29.27, 29.29, 29.45, 29.53, 29.59, 30.80, 31.65, 31.85, 31.89, 32.16, 38.39, 47 64.49, 71.53, 171.87, 173.43. ESI-MS calculated for C 34 h 68 NO 4 + [M+H] + 554.5, found 554.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com