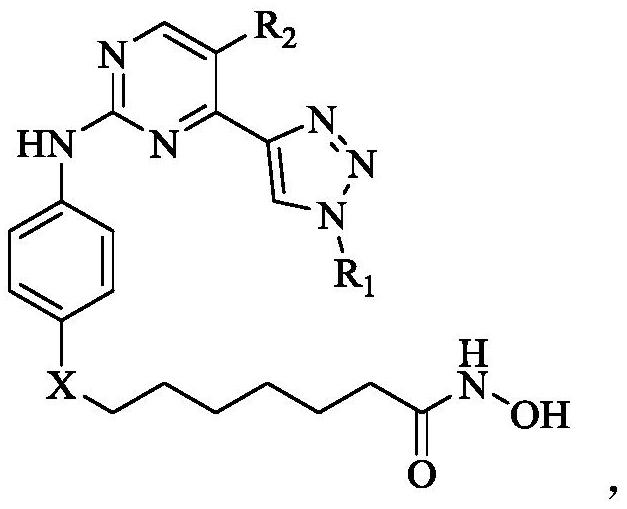
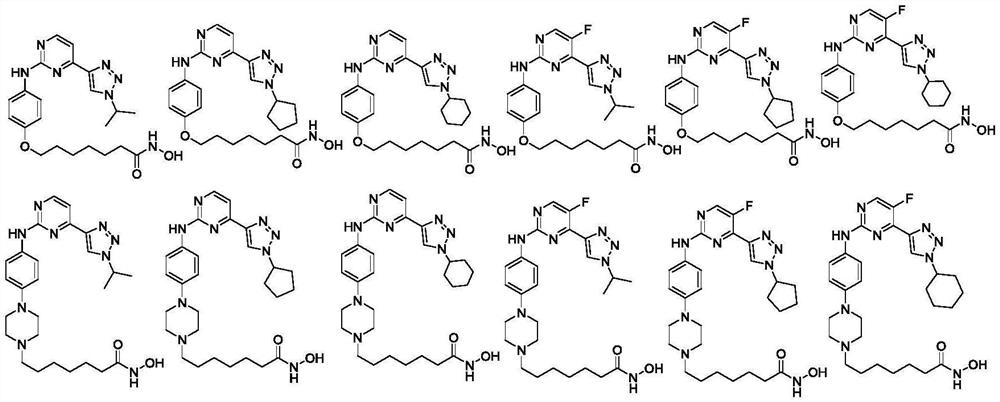
Hydroximic acid-containing 2-phenylaminopyrimidine derivatives and application thereof
A technology of anilinopyrimidine and hydroxamic acid, which is applied to 2-phenylaminopyrimidine derivatives and in the fields of tumor treatment and medicinal chemistry, can solve the problems of large toxic and side effects, difficulty in suppressing tumor development, and easy generation of drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
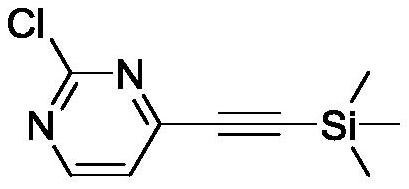
[0034] Example 1: 2-Chloro-4-((trimethylsilyl)ethynyl)pyrimidine (2a)
[0035]
[0036] Triphenylphosphine palladium dichloride (Pd(PPh 3 ) 2 Cl 2 , 0.047g, 0.067mmol), triphenylphosphine (PPh 3 , 0.034g, 0.13mmol), 2,4-dichloropyrimidine (2.0g, 13.4mmol) were added to tetrahydrofuran (THF, 10mL) and triethylamine (Et 3 N, 15 mL) in a mixed solvent, the air in the reaction system was replaced with nitrogen, and then copper iodide (CuI, 0.026 g, 0.13 mmol) and trimethylsilylacetylene (1.579 g, 16.1 mmol) were added in sequence. Cool to room temperature after heating to reflux reaction 3h, filter and remove the solid (Et 3 N . HCl), the filtrate was concentrated, and purified by column chromatography (petroleum ether: ethyl acetate=100:1, volume ratio) to obtain a yellow solid, namely 2-chloro-4-((trimethylsilyl)ethynyl)pyrimidine ( 2a), yield: 57%.
Embodiment 2
[0037] Example 2: 2-Chloro-5-fluoro-4-((trimethylsilyl)ethynyl)pyrimidine (2b)
[0038]
[0039] The preparation method refers to Example 1, wherein 2,4-dichloropyrimidine is replaced by 2,4-dichloro-5-fluoropyrimidine, and the reaction time is 10 minutes. Yellow solid (2b), yield: 62%. 1 H NMR (400MHz, CDCl 3 )δ8.50(s,1H),0.31(s,9H).ESI-MS m / z:229.0[M+H] + .
Embodiment 3
[0040] Example 3: 2-Chloro-4-ethynylpyrimidine (3a)
[0041]
[0042]2-Chloro-4-((trimethylsilyl)ethynyl)pyrimidine (2a, 1.0 g) was dissolved in 10 mL of methanol (MeOH). The KOH solution prepared by dissolving potassium hydroxide (KOH, 3 mg) in 5 mL of methanol was added to the above reaction system, reacted at room temperature for 0.5 h, concentrated and purified by column chromatography (eluted with pure dichloromethane) to obtain a white solid (3a), Yield: 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com