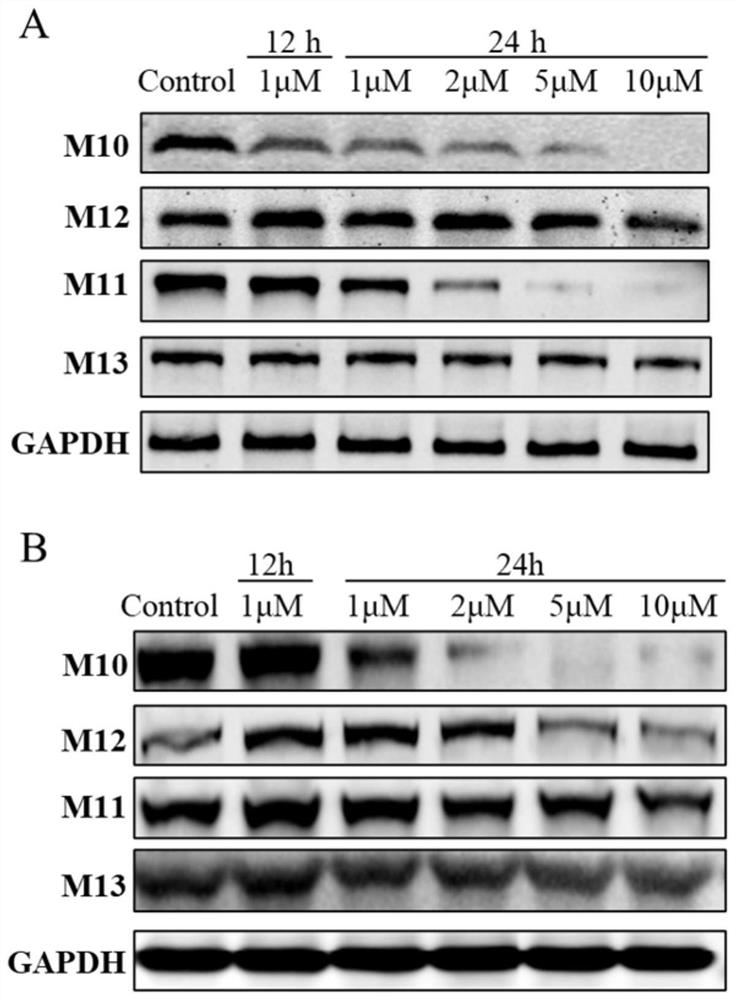
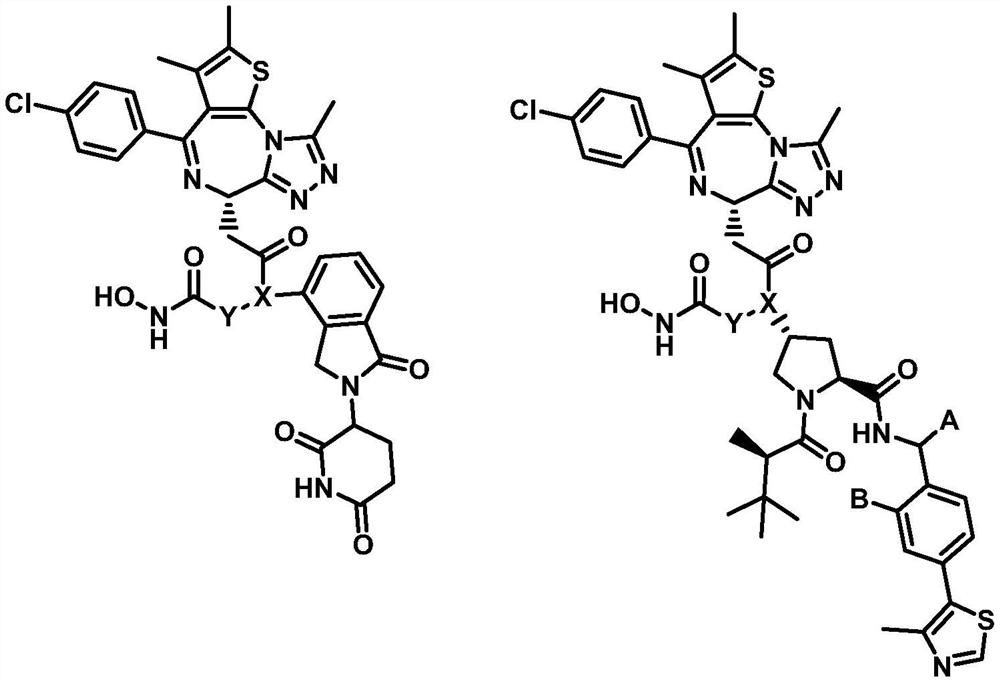
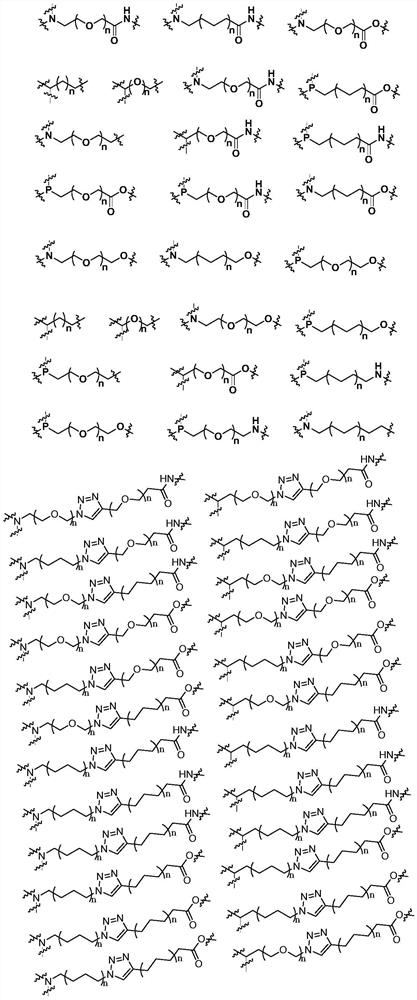
Preparation method and application of protein degradation targeting chimera
A technology of protein degradation and chimera, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 8-(2-((S)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazole [4,3-a][1,4]diazepin-1-yl)-N-(2-(2-(2-(2-((2-(2,6-dioxopiperidine- Synthesis of 3-yl) 1-oxoisoindol-4-yl) amino)-2-oxoethoxy) ethoxy) ethoxy) ethyl) acetamido)-N-hydroxyoctylamide ( M10)
[0056] Step a: tert-butyl (2-(2-(2-(2-(2-((2-(2,6-dioxopiperidin-3-yl)1-oxoisoindolyl-4 Synthesis of -yl)amino)-2-oxoethoxy)ethoxy)ethoxy)urethane (M2)
[0057] Step a: Compound M0 (1.0g, 3.1mmol) H, ATU (2.4g, 6.2mmol) D, IPEA (1.0 mL, 7.8mmol) were dissolved in dry DMF, reacted at room temperature for 0.5h and then added Nalidomide M1 (0.80 g, 3.1 mmol) was reacted for 4 h, and the reaction was complete as detected by TLC. The reaction solution was slowly poured into a mixture of ice water (1.5L), extracted with EA (30mL×3), and the organic phases were combined, and the solvent was evaporated under reduced pressure, and the residue was separated by silica gel column chromatography, and the mobile phase ...
Embodiment 2
[0073] Example 2: Test of the inhibitory activity (Ki) of the compounds of the present invention on BRD4 and HDAC1.
[0074] Add 5 μL of the compound to be tested (each diluted concentration), BRD4 (20 nM) and PMDM6-F (20 nM) (buffer: 100 mM tripotassium phosphate, pH = 7.5; 100 μg / mL BGG; 0.02% sodium azide) into 96-well black The flat-bottom microplate plate was raised to a final volume of 115 μL, and after incubation at room temperature for 1 hour, the fluorescence polarization value was read with a Biotek-Synergy microplate reader (excitation light: 485nM, emission light: 528nM).
[0075] According to the fluorescence polarization value obtained by the above method, the curve was drawn with Origin 9.0 software, and the protein binding inhibition constant (Ki) was calculated. HDAC1 test method is the same as BRD4.
[0076] Experimental results: First, the inhibitory activity of all target compounds on BRD4 and HDAC1 proteins was tested, and (+)-JQ1 and SAHA were selected as ...
Embodiment 3
[0079] Embodiment 3: the in vitro antitumor activity test (IC of compound of the present invention) 50 ).
[0080] The compound of the present invention is tested for three kinds of tumor cell proliferation inhibitory ability, and the test method adopts the conventional CCK8 method. For the logarithmic growth phase tumor cells (MCF-7 (human breast cancer cells)), A549 (human lung cancer cells), HepG2 (human liver cancer cells) were digested with trypsin, and then culture medium (DMEM+10%FBS or PRMI1640+10% FBS) to dilute and suspend the cells into a single cell suspension, adjust the cell density to 5×10 4 cells / mL, add 100 μL to each well and inoculate in a 96-well plate, place at 37°C, 5% CO 2 Cultivate in the incubator for 24 hours, and then add different concentrations of compounds, each concentration of three parallel wells, and set the experimental group and control group, continue to incubate for 72 hours, add 10 μL of CCK8 solution to each well, and then at 37 ° C A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com