Aziridine cross-linked N-halamine type antibacterial PVA sponge as well as preparation method and application thereof
An aziridine cross-linking agent and aziridine technology are applied in the directions of pharmaceutical formulations, pharmaceutical sciences, absorbent pads, etc., which can solve the problems of poor antibacterial durability, complex production process, poor safety of inorganic antibacterial agents, etc., and achieve long-lasting antibacterial properties. , Overcome the complex process, the effect of good antibacterial properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: N halide type PVA molecular synthesis
[0044] Weigh 10g of PVA1799, add it to 100g of deionized water, heat in a water bath at 80°C, stir until the PVA is dissolved, add 0.5g of chloroacetaldehyde and 0.5g of glyoxylic acid, continue stirring for 3 hours, then add 1.13g of 1-chloro-2,2, 5,5-Tetramethyl-4-imidazolidinone continued to react for 3 hours to obtain N-halamine-type PVA aqueous solution, which was denoted as A1.
[0045] Weigh 10g of PVA2088, add it to 100g of deionized water, heat in a water bath at 80°C, stir until the PVA is dissolved, add 0.75g of chloroacetaldehyde and 0.5g of glyoxylic acid, continue stirring for 3 hours, then add 1.68g of 1-chloro-2,2 , 5,5-Tetramethyl-4-imidazolidinone continued to react for 3h to obtain an N-halamine-type PVA aqueous solution, denoted as A2.
[0046] Weigh 10g of PVA1799, add it to 100g of deionized water, heat in a water bath at 80°C, stir until the PVA is dissolved, add 1.0g of chloroacetaldehyde and...
Embodiment 2
[0049] Embodiment 2: preparation of aziridine cross-linked N-halamine type antibacterial PVA sponge
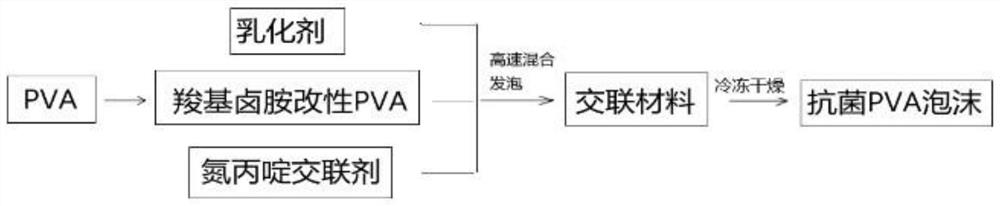
[0050] Add 0.3g emulsifier F68, 0.3g aziridine cross-linking agent trimethylolpropane-tris[3-(2-methyl aziridinyl) propionate] to the A1 solution prepared in the above example 1 , a small high-speed emulsifier with medium-high speed (speed 15000r / min) shear emulsification and cross-linking. After molding, place it in a -70°C refrigerator for 12 hours, and then put it in a freeze dryer for freeze-drying process to obtain N-halamine antibacterial PVA sponge, denoted as S1, the material has an antibacterial rate of 95% against Escherichia coli, 94% against Staphylococcus aureus, and a liquid absorption rate of 19.7g / g.
[0051] It should be noted that in the technology of the present invention, the antibacterial group N-halamine is bound to the PVA molecule through a chemical covalent bond, so that the prepared PVA sponge has durable antibacterial properties.
Embodiment 3
[0052] Embodiment 3: preparation of aziridine cross-linked N-halamine type antibacterial PVA sponge
[0053] Add 0.2g emulsifier F68, 0.3g aziridine cross-linking agent trimethylolpropane-three [3-(2-methyl aziridinyl) propionate] to the A2 solution prepared in the above-mentioned embodiment 1 , a small high-speed emulsifier with medium-high speed (speed 15000r / min) shear emulsification and cross-linking. After molding, place it in a -70°C refrigerator for 12 hours, and then put it in a freeze dryer for freeze-drying process to obtain N-halamine antibacterial PVA sponge, denoted as S2, the material has an antibacterial rate of 97% against Escherichia coli, 96% against Staphylococcus aureus, and a liquid absorption rate of 17.5g / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com