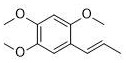
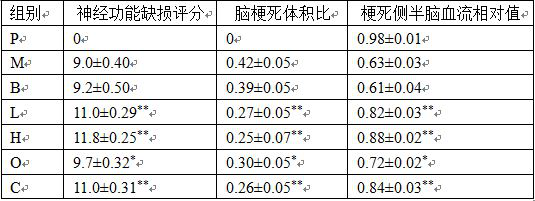
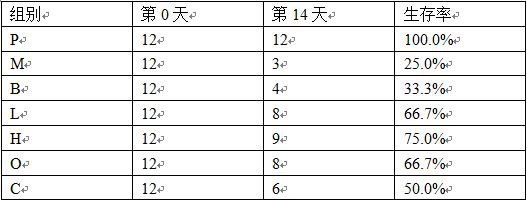
Application of alpha-asarone in preparation of medicine for treating cerebral arterial thrombosis
A technology for ischemic stroke and asarone, which is applied in the field of biomedicine and can solve the problems that the therapeutic effect of α-asarone has not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of α-asarone injection emulsion
[0025] Weigh 0.50~50.0g of α-asarone and 50.0~200.0g of soybean oil for injection, put them in a suitable container, heat to 60~80°C under the protection of nitrogen gas, stir to dissolve; continue to weigh 6.0 g of egg yolk lecithin ~18.0g, add it, stir to dissolve (if necessary, add 0.10~0.50g of oleic acid, sodium oleate or a mixture of the two), and obtain the oil phase, set aside. Separately weigh 0~3.0g of Pluronic F-68, 0~25.0g of glycerol, measure about 800mL of water, heat to 60~80°C under the protection of nitrogen gas, stir to dissolve; the aqueous phase is obtained. Add the above oil phase to the water phase, shear at high speed for 5-15 minutes, add water to 1000mL, and prepare colostrum. Continue to homogenize the colostrum with a high-pressure homogenizer for 3 to 6 times, so that the average particle size of the milk droplets after homogenization is not greater than 0.5 μm, filter through a filter ...
Embodiment 2
[0026] Example 2 Preparation of α-asarone injection emulsion
[0027]Weigh 10.0 g of α-asarone, 50.0 g of soybean oil for injection, and 50.0 g of medium-chain triglyceride (MCT) for injection, put them in a suitable container, heat to 60-80°C under nitrogen protection, and stir to make Dissolving; continue to weigh 12.0 g of egg yolk lecithin and 0.3 g of sodium oleate, add them, stir to dissolve, and obtain an oil phase, which is set aside. Separately weigh 22.0g of glycerin, measure about 800mL of water, heat to 60~80°C under the protection of nitrogen gas, and stir to dissolve; the aqueous phase is obtained. Add the above oil phase to the water phase, shear at high speed for 5-15 minutes, add water to 1000mL, and prepare colostrum. Continue to homogenize the colostrum with a high-pressure homogenizer for 3 to 6 times, so that the average particle size of the milk droplets after homogenization is not greater than 0.5 μm, filter the membrane, and fill the filtrate into 5mL ...
Embodiment 3
[0028] Example 3 Preparation of α-asarone injection emulsion
[0029] Weigh 20.0 g of α-asarone, 100.0 g of soybean oil for injection, and 100.0 g of medium-chain triglyceride (MCT) for injection, put them in a suitable container, heat to 60-80°C under nitrogen protection, and stir to make Dissolving; continue to weigh 12.0 g of egg yolk lecithin and 0.3 g of oleic acid, add it, stir to dissolve, and obtain an oil phase for later use. Separately weigh 22.0g of glycerin, measure about 800mL of water, heat to 60~80°C under the protection of nitrogen gas, and stir to dissolve; the aqueous phase is obtained. Add the above oil phase to the water phase, shear at high speed for 5-15 minutes, add water to 1000mL, and prepare colostrum. Continue to homogenize the colostrum with a high-pressure homogenizer for 3 to 6 times, so that the average particle size of the milk droplets after homogenization is not greater than 0.5 μm, filter the membrane, and fill the filtrate into 5mL or 10mL ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com