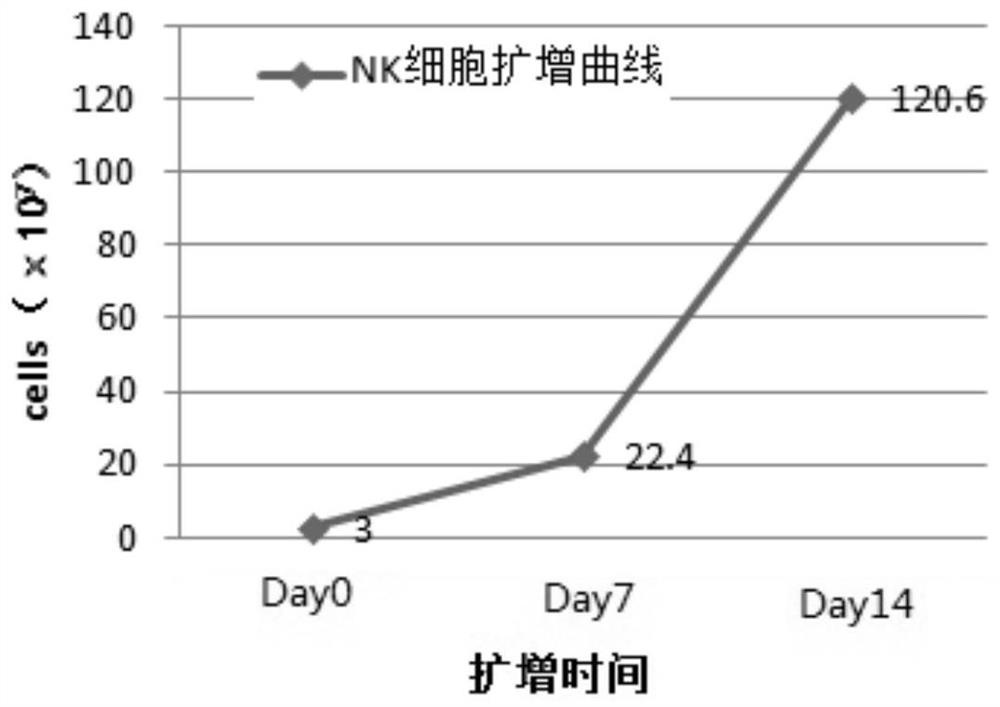
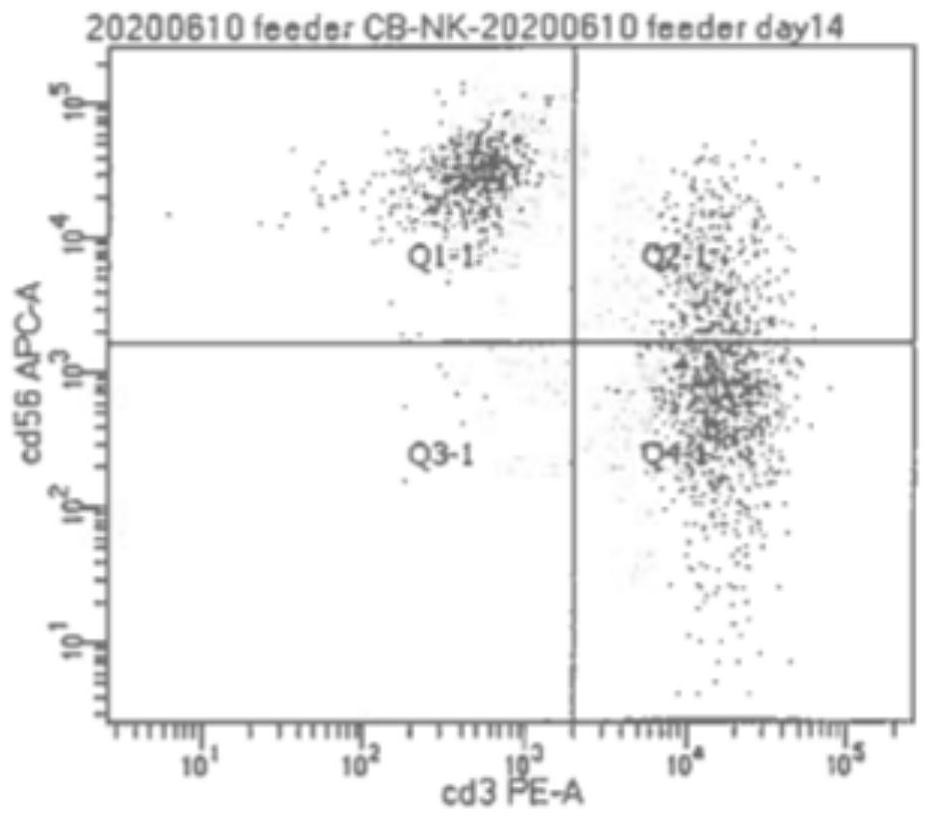
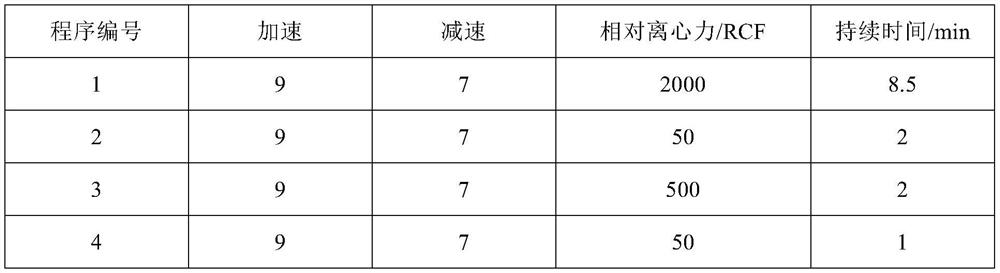
Method for amplifying natural killer cells from placenta tissue
A natural killer cell and tissue technology, applied in the field of bioengineering, can solve the problems of long extraction time, small number of mononuclear cells, and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: the acquisition of placental blood
[0080] 1. Placenta cleaning: Use surgical forceps to take out the placental tissue (50g), put it in a stainless steel tray, and wash the surface of the placenta with a small amount of tissue cleaning solution containing penicillin-streptomycin-amphotericin to remove blood coagulation stains on the surface [In the present invention, if not otherwise stated, the tissue cleaning solution used is to prepare the gained solution in the following way: be mixed with penicillin 100U / ml, streptomycin 0.1mg / ml and 0.25 with 0.9% sodium chloride injection The solution of μg / ml amphotericin B was sterilized by filtration to obtain];
[0081] 2. Use scissors and tweezers to bluntly peel off and discard the amniotic layer on the surface of the placenta, and cut off the umbilical cord tissue above it. Then use scissors to cut the remaining placental lobule tissue into 3-7cm 3 Take a 300-mesh stainless steel filter and place it on t...
Embodiment 2
[0082] Example 2: Digestion of Placental Lobules Tissue
[0083] 1. Configuration of digestive juice:
[0084] 1. Add PBS to the type I collagenase powder, fully mix and dissolve, and configure a type I collagenase solution with a concentration of 10 mg / ml; Salt buffer solution, its preparation method is: take 250ml of 0.2mol / L potassium dihydrogen phosphate solution, add 118ml of 0.2mol / L sodium hydroxide solution, dilute with water to 1000ml, shake well, and obtain];
[0085] ②Add HBSS buffer solution to the type II collagenase powder, fully mix and dissolve, and configure a type II collagenase solution with a concentration of 10 mg / mL; [In the present invention, unless otherwise specified, the HBSS buffer solution used is obtained by The resulting solution was prepared as follows: 8.0 g of NaCl, 0.4 g of KCl, 0.1 g of MgSO4 7H2O, 0.1 g of MgCl2 6H2O, 0.06 g of Na2HPO4 2H2O, 0.06 g of KH2PO4, 1.0 g of glucose, 0.14 g CaCl2, 0.35g of NaHCO3 add distilled water to 1000ml t...
Embodiment 3
[0092] Example 3: Using Extraction of single nucleocytes from placental blood and placental cell suspension by automated separation equipment cell
[0093]1. Mix the placental blood obtained in Example 1 and the placental lobular tissue cell suspension obtained in Example 2, and inject the mixed solution into the 200ml blood collection bag containing anticoagulant 3.2% sodium citrate solution with a sterile syringe. The volume ratio of the coagulant to the biological sample is 1:12, after mixing, place it on a shaker and shake it slowly for 15 minutes;
[0094] 2. will The plastic needle of the disposable separation cup of the automatic separation equipment is inserted into the sterile interface on the blood collection bag, and the blood collection bag is hung up, so that the blood in it flows naturally into the central compartment of the separation cup; the pipeline is welded with a sterile splicer Separate the blood collection bag from the disposable separation cup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com