Ion-selective gel-state electrolyte, preparation method and lithium-sulfur battery
A technology for ion-selective, lithium-sulfur batteries, applied in the field of preparation methods and lithium-sulfur batteries, ion-selective gel electrolytes, can solve the problems of reducing the solubility of polysulfide ions, low Coulombic efficiency, weakening shuttle behavior, etc., to achieve improved Effects of low Coulombic efficiency, promotion of transport, and inhibition of shuttle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
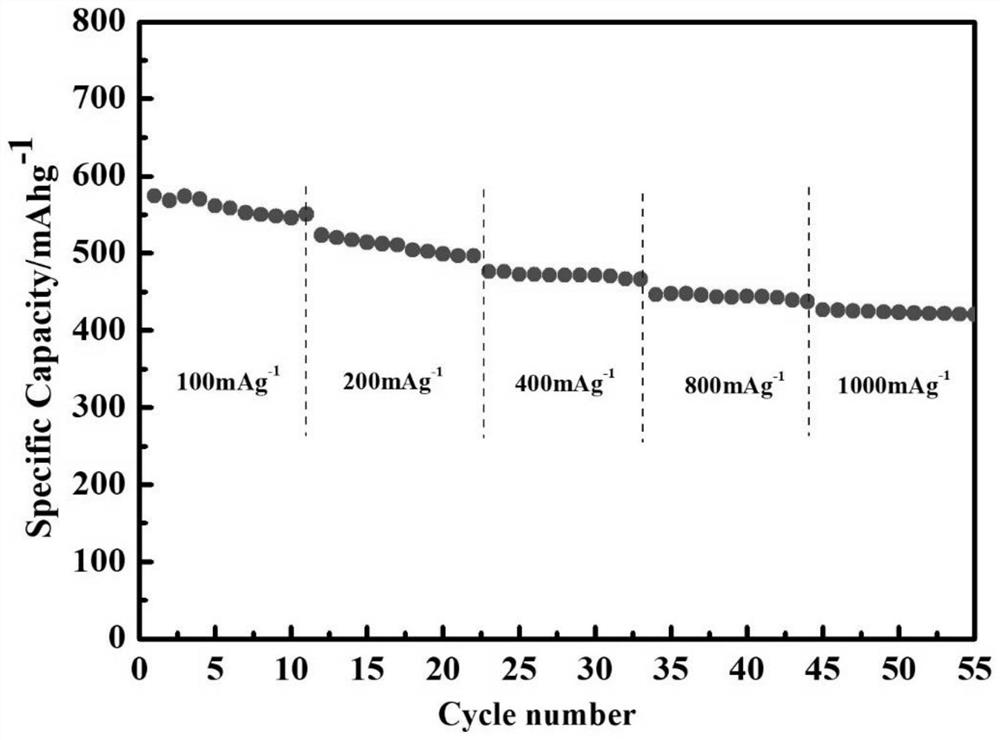
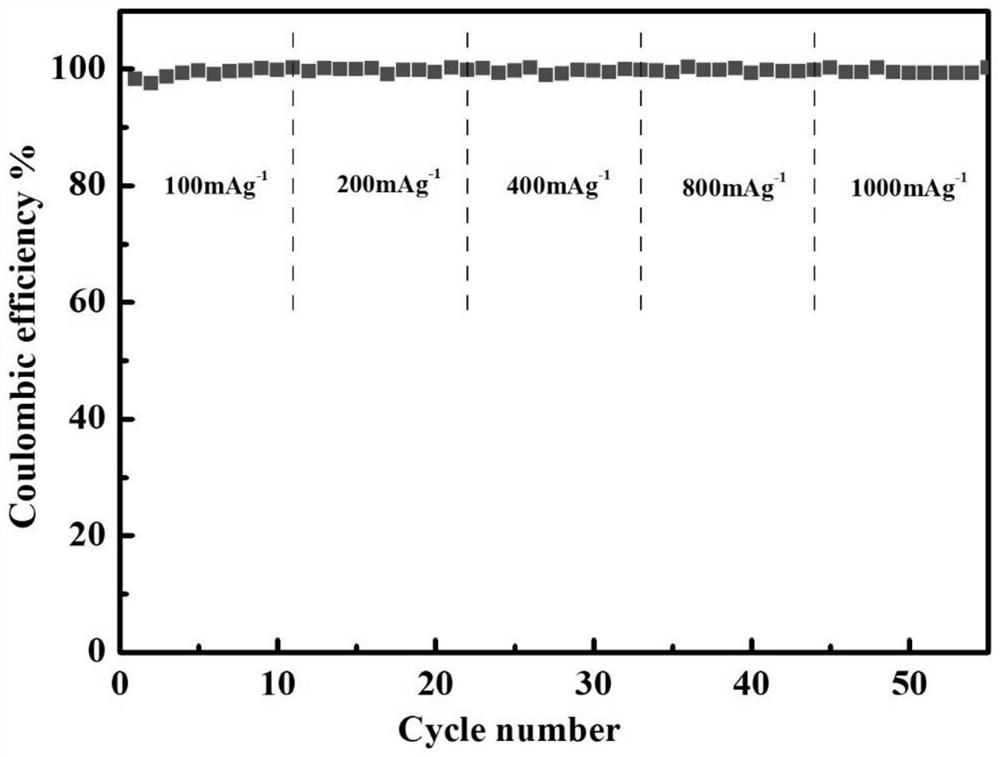
[0037] Mix polyetheretherketone and concentrated sulfuric acid at a mass ratio of 1:40, and react at 55°C for 6h. Add a cross-linking agent with a mass ratio of 1% and sodium trimetaphosphate to continue the reaction for 10 minutes. After the reaction is completed, pour the mixed solution into an ice-water bath to precipitate, filter and wash until the filtrate is neutral, and dry it in a vacuum oven at 50°C. After 24h, cross-linked sulfonated polyetheretherketone was obtained. Weigh cross-linked sulfonated polyetheretherketone dissolved in NMP+H 2 In a mixed solution of O (volume ratio 1:1), the mass concentration of cross-linked sulfonated polyetheretherketone was 4%, and the obtained homogeneous solution was coated on a glass plate to obtain a wet film, and stood at room temperature for 3 hours to defoam. Transfer to a vacuum oven and dry at 50°C for 24 hours to form a film. The dry polymer film was cut into a disc with a diameter of 16 mm, immersed in a lithium-sulfur el...
Embodiment 2
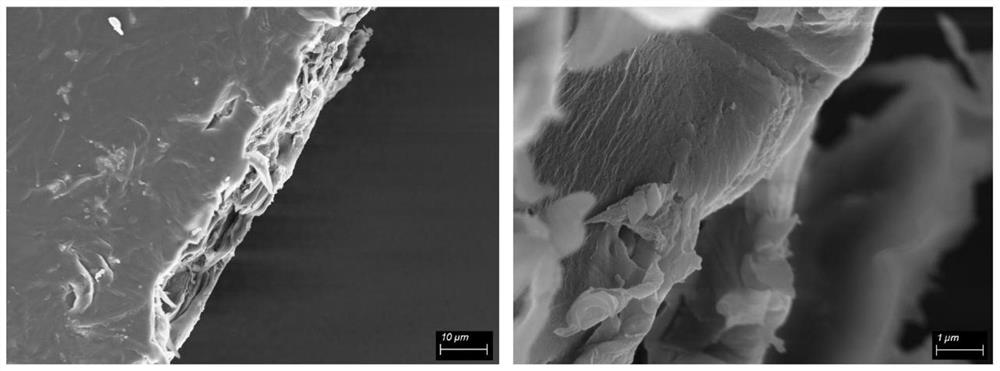
[0039] Mix polyetheretherketone and concentrated sulfuric acid at a mass ratio of 1:50, and react at 65°C for 4h. Add a cross-linking agent with a mass ratio of 30% and sodium trimetaphosphate to continue the reaction for 60 minutes. After the reaction is completed, pour the mixed solution into an ice-water bath to precipitate, filter and wash until the filtrate is neutral, and dry it in a vacuum oven at 90°C. 12h to obtain cross-linked sulfonated polyetheretherketone. Weigh cross-linked sulfonated polyetheretherketone dissolved in DMF+H 2 In a mixed solution of O (volume ratio 1:4), the mass concentration of the cross-linked sulfonated polyetheretherketone was 10%, and the obtained homogeneous solution was coated on a glass plate to obtain a wet film, and stood at room temperature for 6 hours to defoam. Transfer to a vacuum oven and dry at 90°C for 12 hours to form a film. The dry film was characterized by SEM, the surface of the film was relatively smooth, and uniform netw...
Embodiment 3
[0041] Mix polyetheretherketone and concentrated sulfuric acid at a mass ratio of 1:40, and react at 55°C for 6h. Add a cross-linking agent with a mass ratio of 5% and sodium trimetaphosphate to continue the reaction for 30 minutes. After the reaction is completed, pour the mixed solution into an ice-water bath to precipitate, filter and wash until the filtrate is neutral, and dry it in a vacuum oven at 80°C. After 24h, cross-linked sulfonated polyetheretherketone was obtained. Weigh cross-linked sulfonated polyetheretherketone dissolved in NMP+H 2 In a mixed solution of O (volume ratio 1:2), the resulting homogeneous solution was coated on a glass plate to obtain a wet film, left at room temperature for 4 hours to defoam, then transferred to a vacuum drying oven at 80°C for 24 hours to form a film. The dry polymer film was cut into a disc with a diameter of 16 mm, immersed in a lithium-sulfur electrolyte (1M LiTFSI DOL / DME (V / V=1:1)+1% LiNO 3 ), to obtain a polymer electrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com