B/N organic electroluminescent material and preparation method and application thereof
An electroluminescence, electromechanical technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as poor color purity, and achieve low-efficiency roll-off, high-efficiency thermally activated delayed fluorescence, and high fluorescence quantum yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
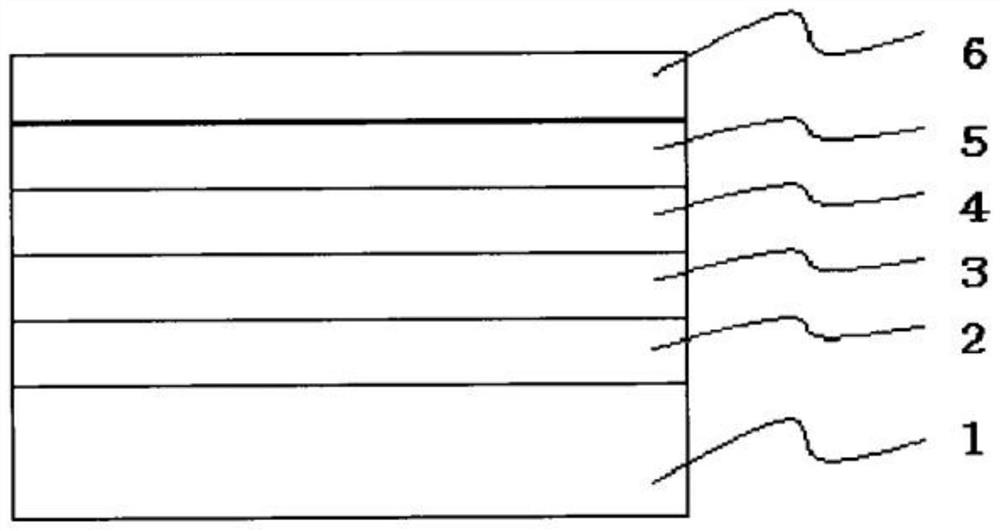
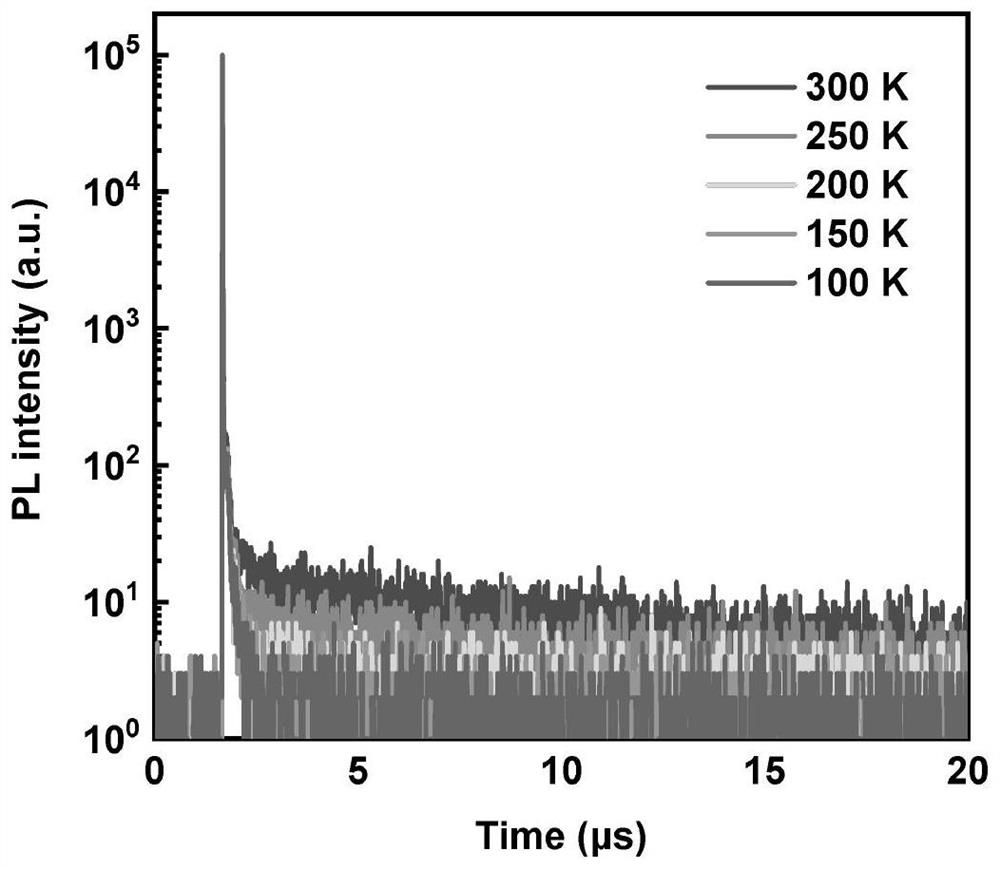
Image
Examples
Embodiment 1
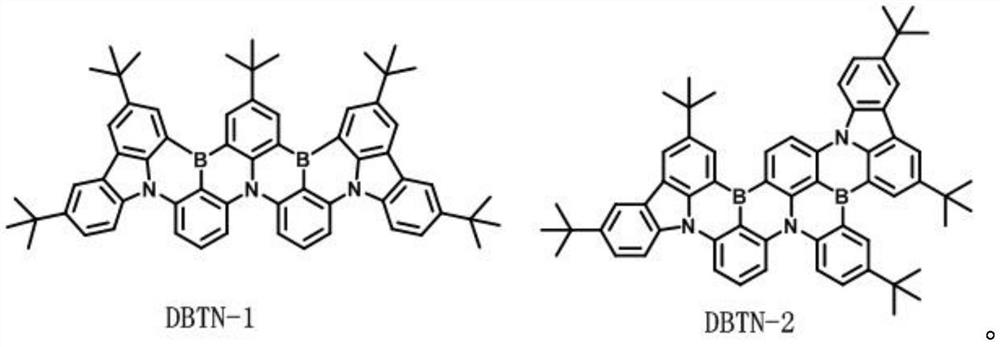
[0027] Embodiment 1: the synthesis of DBTN-1 and DBTN-2
[0028]
[0029] 1. Synthesis of Intermediate 1:
[0030] Under nitrogen protection, 5.6g (20mmol) of 3,6-di-tert-butylcarbazole, 4.2g (20mmol) of 2-fluoro-6-bromochlorobenzene, and 9.8g (30mmol) of cesium carbonate were successively added to a 250mL two-necked flask. And 200mL ultra-dry N,N-dimethylformamide solvent. After nitrogen replacement, the resulting reaction solution was heated to 150° C. and stirred for 24 hours. After the reaction was cooled to room temperature, it was slowly poured into 500 mL of deionized water, stirred and filtered to obtain a white filter cake. Using PE:DCM (6:1) as the eluent, it was further purified by silica gel column chromatography to obtain 9.0 g of pure white solid with a yield of 96%.
[0031] Product characterization: 1 H NMR (600MHz, CDCl 3 -d) δ8.16(dd, J=3.5,1.8Hz,2H),7.80(dt,J=8.1,1.2Hz,1H),7.45(ddt,J=8.2,6.1,1.7Hz,3H),7.31 (t,J=8.0Hz,1H),7.00(dd,J=8.5,2.0Hz,2H),1.47...
Embodiment 2
[0039] 1. Synthesis of intermediate 1:
[0040] Under nitrogen protection, 5.6 g (20 mmol) of 3,6-di-tert-butylcarbazole, 4.2 g (20 mmol) of 2-fluoro-6-bromochlorobenzene, and potassium tert-butoxide (30 mmol) were successively added to a 250 mL two-necked flask. And 200mL ultra-dry N,N-dimethylformamide solvent. After nitrogen replacement, the resulting reaction solution was heated to 150° C. and stirred for 24 hours. After the reaction was cooled to room temperature, it was slowly poured into 500 mL of deionized water, stirred and filtered to obtain a white filter cake. Using PE:DCM (6:1) as eluent, it was further purified by silica gel column chromatography to obtain 9.6 g of pure white solid.
[0041] 2. Synthesis of intermediate 2:
[0042] Under nitrogen protection, 5.2g intermediate 1 (11mmol), 4-tert-butylaniline 746mg (5mmol), palladium acetate (0.5mmol), tri-tert-butylphosphine tetrafluoroborate 290mg ( 1mmol), sodium tert-butoxide 2.0g (30mmol) and 150mL ultra-d...
Embodiment 3
[0045] 1. Synthesis of intermediate 1:
[0046] Under nitrogen protection, 5.6 g (20 mmol) of 3,6-di-tert-butylcarbazole, 4.2 g (20 mmol) of 2-fluoro-6-bromochlorobenzene, and sodium tert-butoxide (30 mmol) were successively added to a 250 mL two-necked flask. and 200 mL of ultra-dry meta-xylene solvent. After nitrogen replacement, the resulting reaction solution was heated to 150° C. and stirred for 24 hours. After the reaction was cooled to room temperature, it was slowly poured into 500 mL of deionized water, stirred and filtered to obtain a white filter cake. Using PE:DCM (6:1) as the eluent, it was further purified by silica gel column chromatography to obtain 8.5 g of pure white solid.
[0047] 2. Synthesis of intermediate 2:
[0048] Under the protection of nitrogen, 5.2g of intermediate 1 (11mmol), 746mg (5mmol) of 4-tert-butylaniline, palladium chloride (0.5mmol), and 290mg of tri-tert-butylphosphine tetrafluoroborate were successively added to a 250mL two-necked r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com