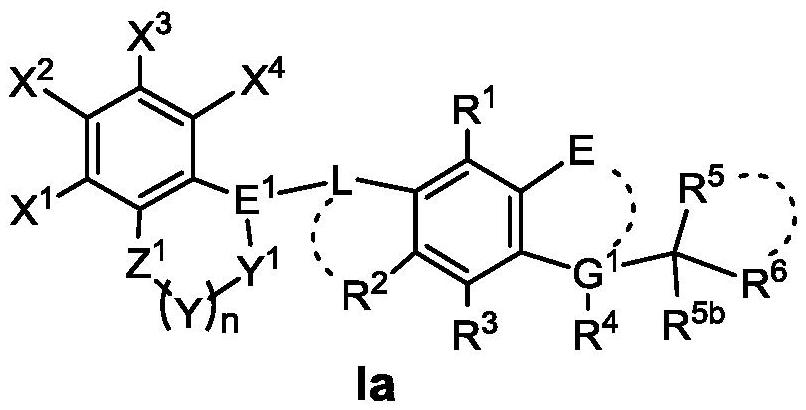
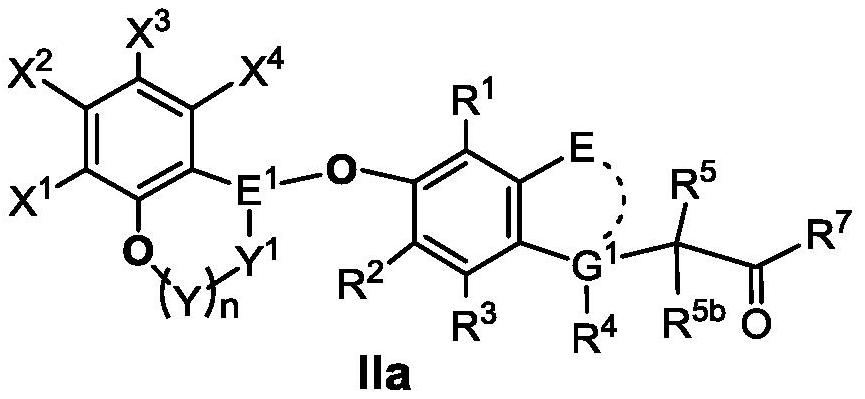
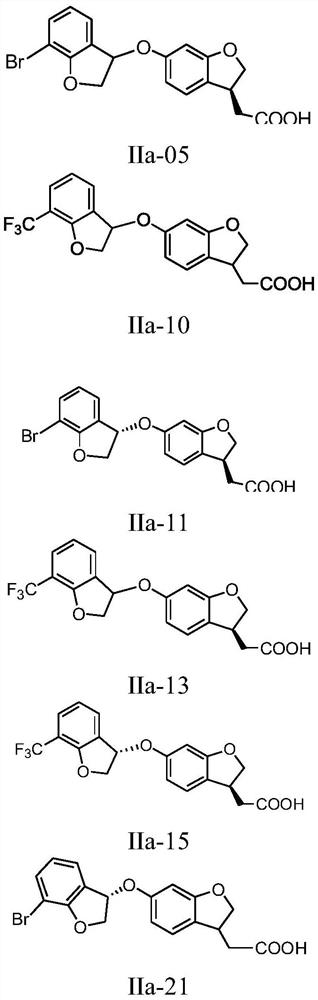
Benzo oxygen-containing heterocyclic compound and medical application thereof
A technology of compounds and hydrates, applied in the field of novel benzoxygen-containing heterocyclic compounds, can solve problems such as liver toxicity and unsafe molecular structure design, and achieve lower blood sugar levels, lower risk of hypoglycemia, good selectivity and safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] Synthesis of Compound IIa-1
[0250] The first step: raw materials RM-Ia-1 (0.052g, 0.24mmol.), SM1-1 (0.050g, 0.24mmol, 1.0eq.) and PPh3 (0.126g, 0.48mmol, 2eq.) were dissolved in 2L In THF, under nitrogen protection, under cooling in an ice-water bath, add DIAD (0.097g, 0.48mmol, 2eq.) while stirring. After the addition, stir in an ice-water bath for 1h at 20-30°C, react overnight, and the reaction is complete. After HPLC showed that the reaction was finished, the reaction solution was cooled to room temperature, water was added, DCM was added to extract, and the organic phases were combined, washed with brine, dried, and concentrated to obtain a crude product; the crude product was separated by a TLC scraper to obtain an intermediate product RM-IIa-1 (0.029g);
[0251] Confirmed by mass spectrometry, the ESI-MS [(M+H) of RM-IIa-1 + ]: m / z theoretical value 405.0, measured value 405.0.
[0252] The second step: add MeOH (2mL) to the intermediate product RM-IIa-1 (0...
Embodiment 2
[0255] Synthesis of Compound IIa-2
[0256] The synthetic method for preparing compound IIa-2 is the same as in Example 1, and the product IIa-2 is obtained through two steps of etherification and hydrolysis, wherein compound RM-Ia-2 (0.23 mmol) is used in the reaction instead of compound RM-Ia-4 The intermediate RM-IIa-2 was obtained, and the light yellow solid product IIa-2 (0.016g) was obtained by hydrolysis of the intermediate, the two-step yield: 17.8%.
[0257]Confirmed by mass spectrometry, ESI-MS of IIa-2 [(M-H) + ]: m / z theoretical value 389.0, measured value 389.0.
Embodiment 3
[0259] Synthesis of Compound IIa-3
[0260] The synthetic method for preparing compound IIa-3 is the same as in Example 1, and the product IIa-3 is obtained through two-step reaction of etherification and hydrolysis, wherein compound RM-Ia-3 (0.24mmol) is used to replace compound RM-Ia-4 in the reaction The intermediate RM-IIa-3 was obtained, and the product IIa-3 (0.030g) was obtained as a light yellow solid by hydrolysis of the intermediate, and the two-step yield: 32%.
[0261] Confirmed by mass spectrometry, ESI-MS of IIa-3 [(M-H) + ]: m / z theoretical value 389.0, measured value 389.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com