Application of chlamydia protein Pgp3 in preparation of medicine for inhibiting salpingitis
A chlamydia protein, fallopian tube technology, applied in the field of biomedicine, can solve problems such as ambiguity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
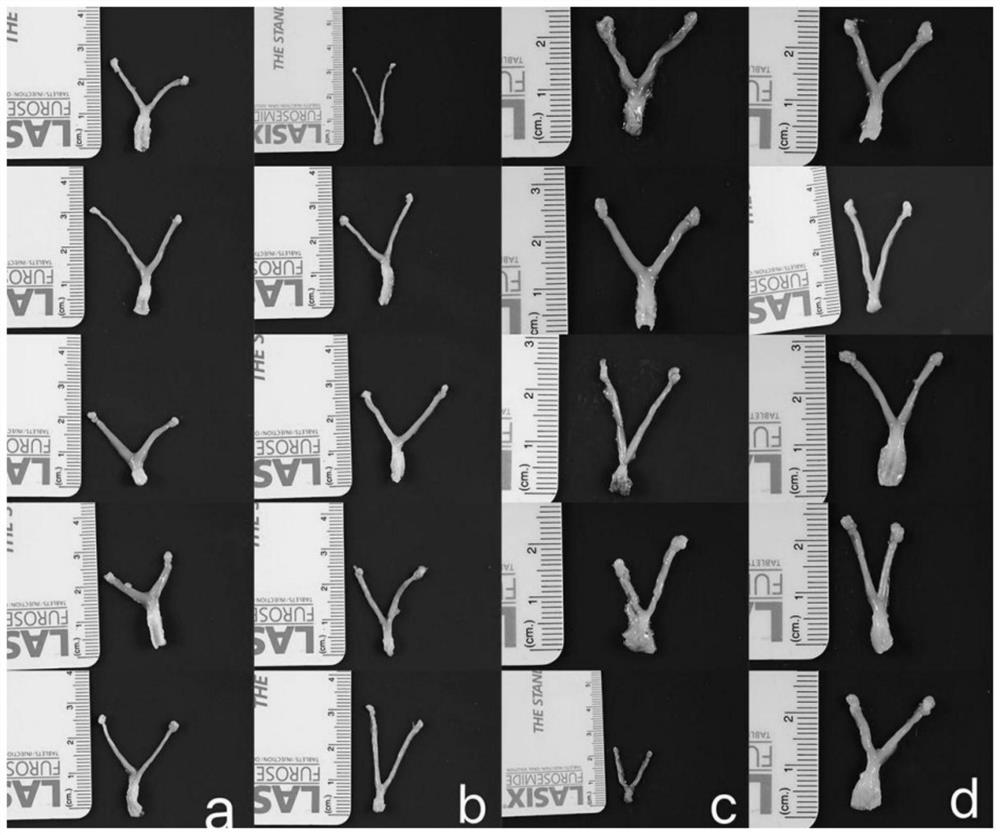
[0036] The construction of embodiment 1 experimental mouse model
[0037] 1.1 Preparation of experimental animals
[0038] 20 C3H / HeJ female mice of SPF grade, weighing 18-20 g and aged 5-6 weeks were raised.
[0039] 1.2 Experimental grouping and intervention
[0040] 20 mice were randomly divided into 4 groups, 5 in each group, i.e. blank group (without any treatment), negative control group (i.e. CT795 group), high concentration group (His-pgp3 concentration is 10 μg / μl), low concentration group (His-pgp3 concentration was 1 μg / μl).
[0041] (1) Dilute the purified His-pgp3 to 10 μg / μl and 1 μg / μl with sterile PBS liquid.
[0042] (2) Inject His-pgp3 (10 μg / μl, 10 μl) into the left and right ovaries of each mouse in the high concentration group, that is, inject 100 μg of His-pgp3 into the left and right ovaries of each mouse.
[0043] (3) Inject His-pgp3 (1 μg / μl, 10 μl) into the left and right ovaries of each mouse in the low concentration group, that is, inject 10 μg ...
Embodiment 2
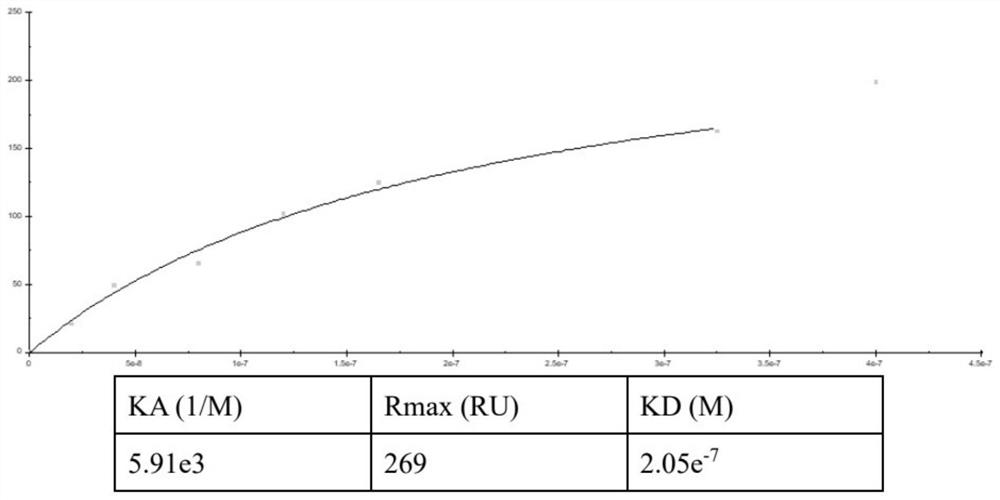
[0052] Example 2 Affinity Detection of His-pgp3, TNF-α and TNFR1
[0053] Using the amino acid coupling method, the diluted His-Pgp3 was immobilized on the activated CM5 chip. TNF-α and TNFR1 were injected into the channel with different concentration gradients, and the signal intensity was detected. All signals are corrected via a blank channel. Analytical processing was performed with Biacore 3000 Control Software.
[0054] image 3 Kinetic analysis diagram of His-pgp3 pair coupled antibody TNF-α, Figure 4 Kinetic analysis diagram of His-pgp3 pair coupled antibody TNFR1, by image 3 with Figure 4 It can be seen that as the concentration of TNF-α increases, the signal value increases, with a certain saturation trend, and the KD(M) value obtained by fitting is 2.05e-7( image 3 ), but as the concentration of TNFR1 increased, the signal value increased linearly without a saturation trend. Prove that His-Pgp3 and TNFR1 are non-specific binding ( Figure 4 ).
Embodiment 3
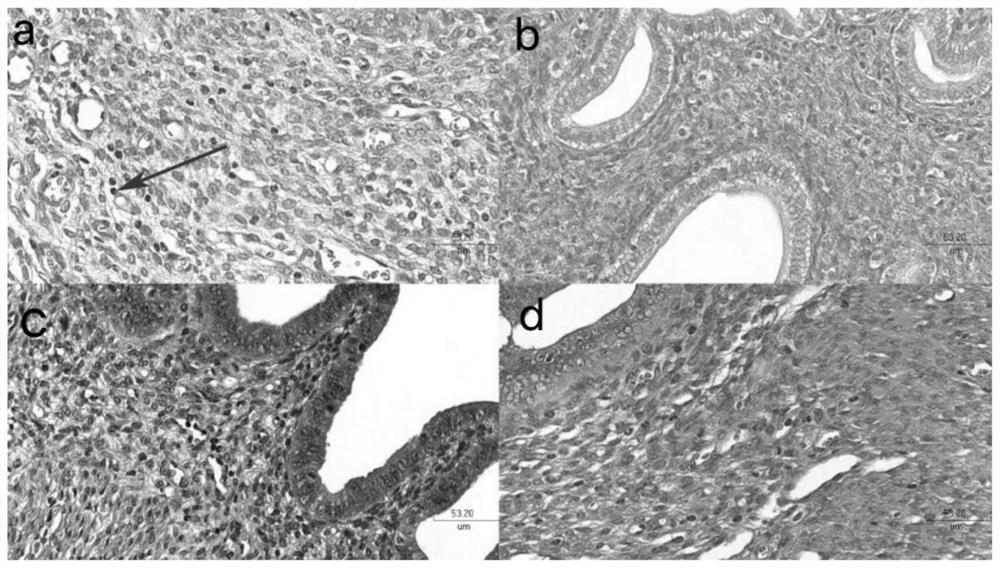
[0055] Example 3 Cell Apoptosis Rate Detection
[0056] 1.Hoechest 33258 detection of apoptosis rate
[0057] Remove the old culture medium in the 24-well plate, add 1ml PBS buffer to each well to wash the cells 2-3 times, remove the PBS buffer; add 4% paraformaldehyde to each well to fix for 15-20min; wash with PBS buffer 3 times, each time 3 to 5 minutes each time; Dilute Hoechst 33258 stock solution 100 times with physiological saline or PBS buffer solution, which is the working solution. Add working solution to the 24-well plate, 200 μl working solution per well, and stain in the dark for 3-5 minutes at room temperature; remove Hoechst 33258 staining solution, wash with PBS buffer three times, 3-5 minutes each time; observe cell apoptosis under a fluorescent microscope death situation.
[0058] Experimental results such as Figure 5 As shown, compared with the positive control group, most of the nuclei in the mixed group were uniformly light blue ( Figure 5 The middle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com