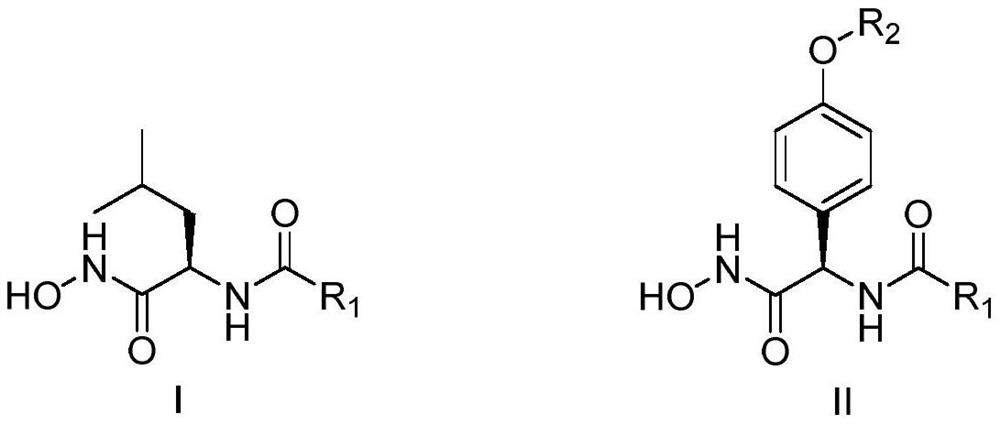
D-amino acid derivative aminopeptidase N inhibitor as well as preparation method and application thereof
A technology of derivatives and amino acids, applied in anti-inflammatory agents, drug combinations, pharmaceutical formulations, etc., can solve problems such as poor drugability and single compound structure, and achieve rich structural diversity, simple operation, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
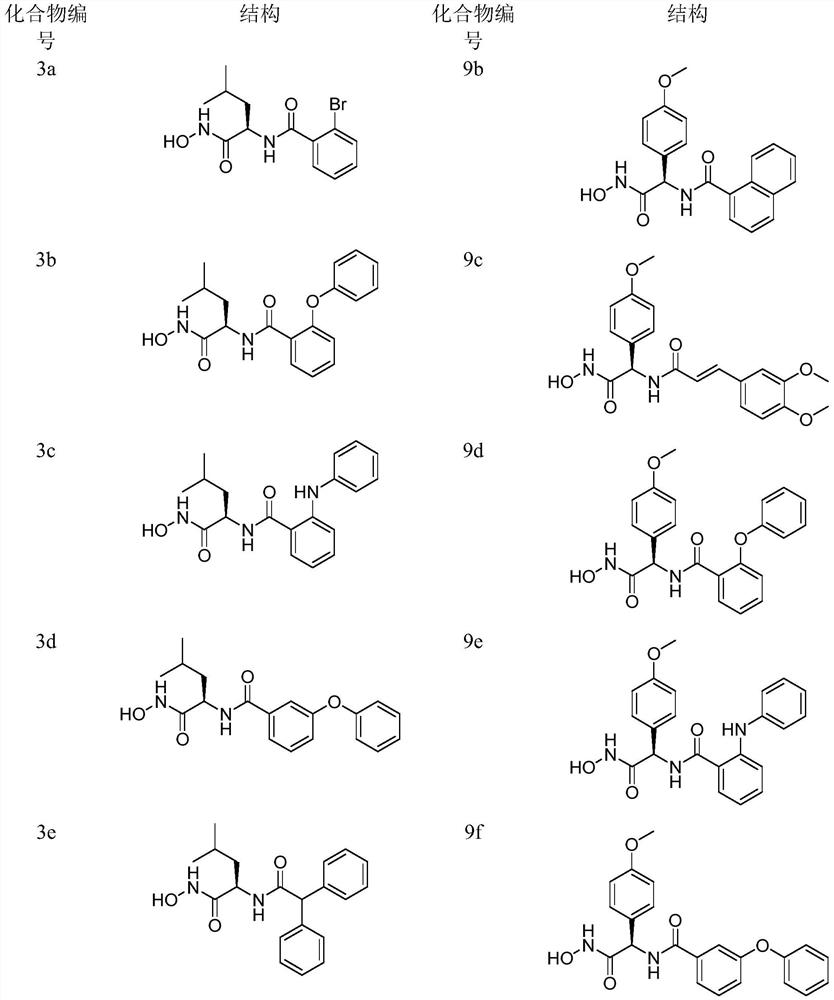
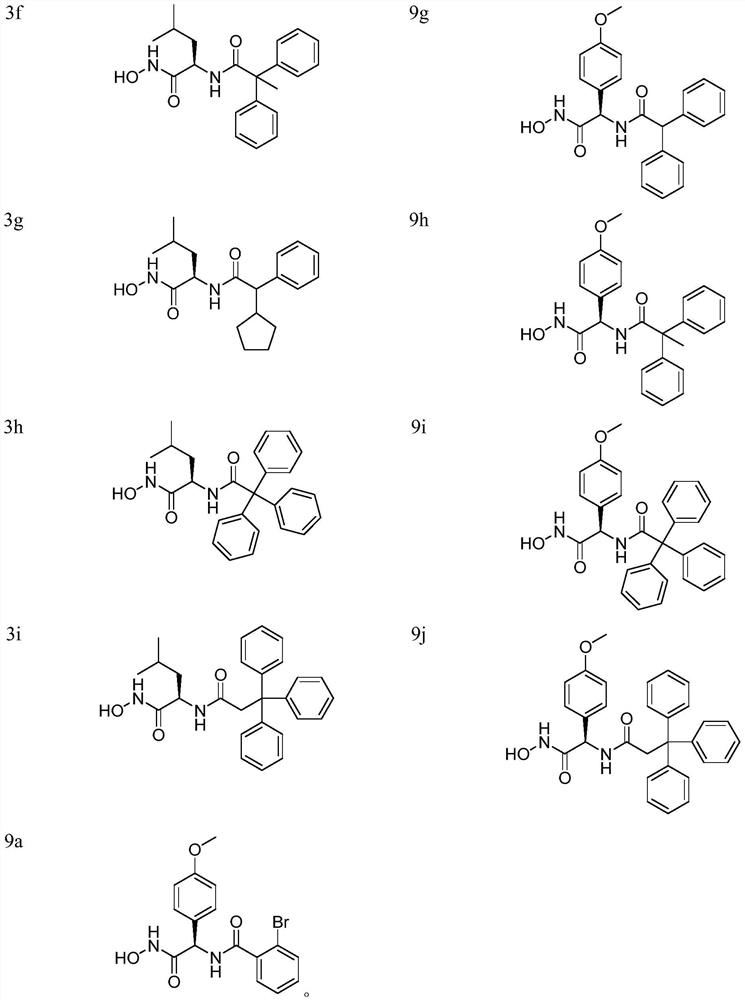
[0088] Example 1. The preparation of compounds 3a-3i, taking 3h as an example.
[0089] The preparation method of (R)-N-hydroxyl-4-methyl-2-(2,2,2-triphenylacetamido)pentanamide (3h), the steps are as follows:
[0090] 1) Preparation of methyl (2,2,2-triphenylacetyl)-D-leucine (2h)
[0091] 2,2,2-Triphenylacetic acid (0.5g, 1.74mmol) was dissolved in anhydrous dichloromethane (10mL), at 0°C, 1-ethyl-(3-dimethylaminopropyl)carbon was added Imide hydrochloride (0.38g, 1.98mmol), 1-hydroxybenzotriazole (0.26g, 1.93mmol) and 4-dimethylaminopyridine (0.04g, 0.33mmol). After reacting at 0°C for 30 minutes, add D-leucine methyl ester hydrochloride (0.34g, 1.87mmol) and triethylamine (0.22g, 2.17mmol); react at 25°C for 12 hours; dichloromethane was concentrated and the residue Add ethyl acetate (20 mL), wash with 10% phosphoric acid (20 mL) three times, once with saturated aqueous sodium bicarbonate (20 mL), once with saturated aqueous sodium chloride (20 mL), and dry over anhydrou...
Embodiment 2
[0111] Example 2. The preparation of compounds 9a-9j, taking 9i as an example;
[0112] (R)-N-(2-(hydroxylamino)-1-(4-methoxyphenyl)-2-oxoethyl)-2,2,2-triphenylacetamide (9i) Preparation method, the steps are as follows:
[0113] 1) Preparation of methyl (R)-2-((tert-butoxycarbonyl)amino)-2-(4-hydroxyphenyl)acetate (5)
[0114] (R)-4-Hydroxyphenylglycine methyl ester hydrochloride (6.51g, 30mmol) was dissolved in dichloromethane (50mL), and triethylamine (7.58g, 75mmol) was added at 0°C. At 0℃, add dropwise (Boc) 2 O (7.84 g, 36 mmol) in dichloromethane (20 mL). After the dropwise addition was completed, the reaction was carried out at 25° C. for 24 hours. Dichloromethane was concentrated, and the residue was dissolved in ethyl acetate (100 mL), washed three times with 10% phosphoric acid (50 mL), once with saturated aqueous sodium bicarbonate (50 mL), and once with saturated aqueous sodium chloride (50 mL). Second-rate. Purification by column chromatography: volume rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com