Synthetic method of emamectin benzoate
A synthetic method and emamectin benzoate technology, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation, etc., to achieve the effects of accurate feeding, stable continuous automation, and less material volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] A kind of synthetic method of emamectin benzoate, comprises the following steps:
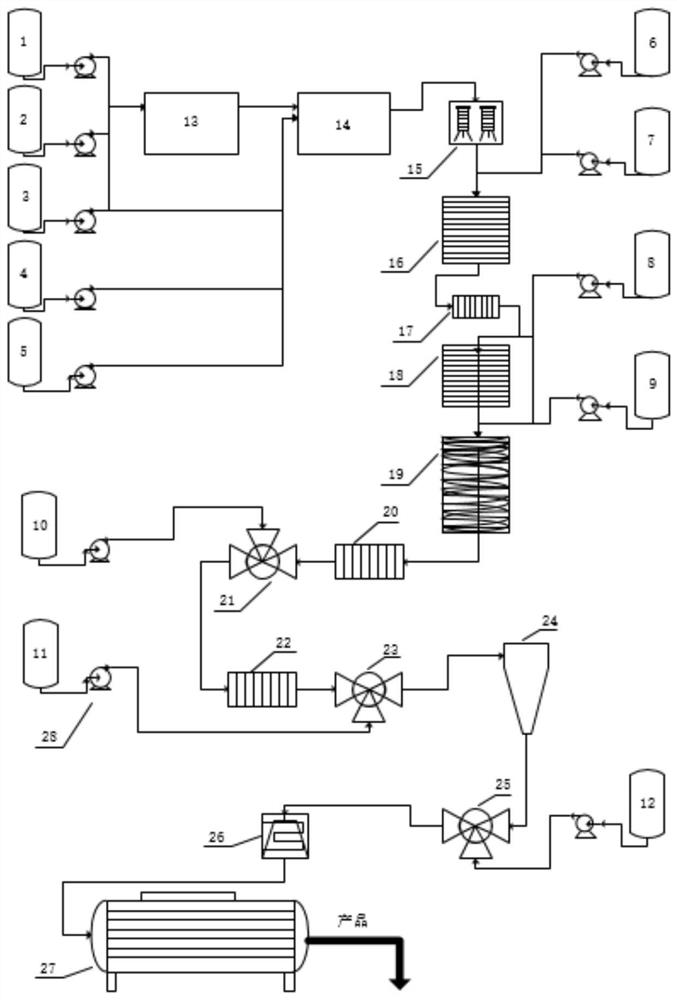
[0069] (1) Mix avermectin B1 and dichloromethane in a mass ratio of 1:3 and add them to the dissolving kettle, fully stir and dissolve and lower the temperature to -30°C to obtain the abamectin solution, which is transferred to the abamectin solution Vermectin solution storage tank (1) is stand-by.
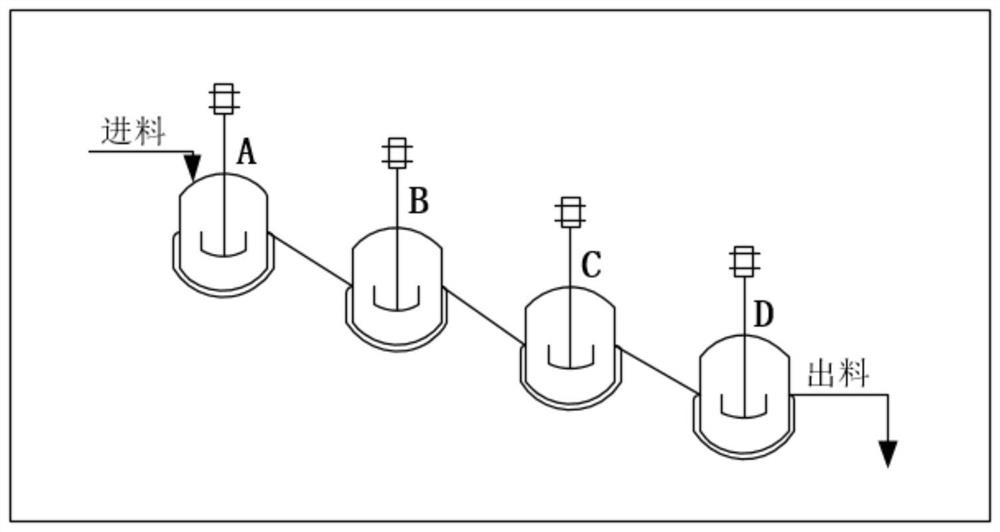
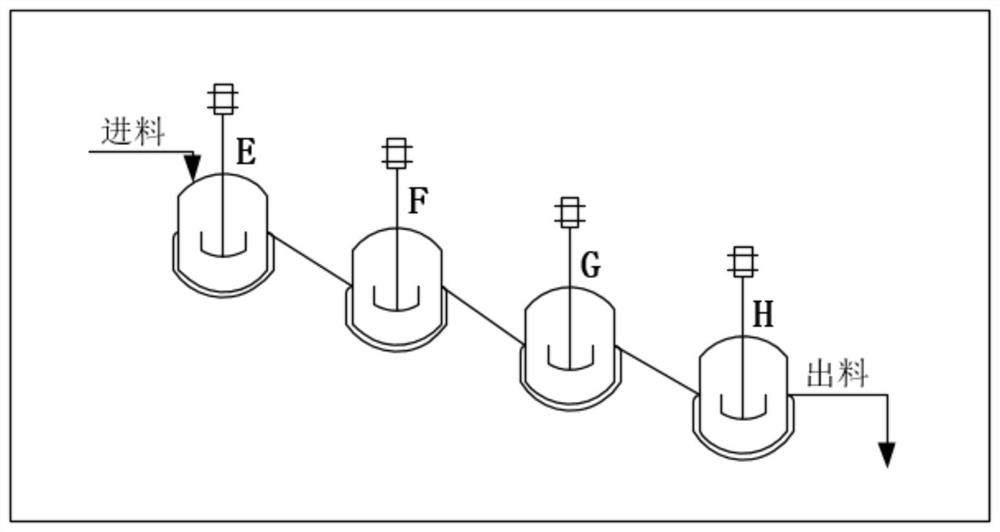
[0070] Control the reaction temperature in the protection tank (A / B / C / D) to be -30±5°C. The Abamectin solution prepared in the step (1) enters the A still according to the flow rate of 520Kg / h, and simultaneously pumps allyl chloroformate (protective agent) and tetramethylethylenediamine (alkali) into the A still, The flow rates of the two are controlled at 5.85Kg / h and 10.38Kg / h respectively. After the three materials are fully mixed and reacted in the A tank; the feed liquid enters the B tank through self-flow, and at the same time pumps allyl chloroformate, the flow rate is controlled at...
Embodiment 2
[0083] A kind of synthetic method of emamectin benzoate, comprises the following steps:
[0084] The difference from Example 1 is that,
[0085] Protection process: the protective agent is ethyl chloroformate, the organic base is triethylamine, the molar ratio of abamectin and triethylamine is 1:0.8, and the molar ratio of avermectin and ethyl chloroformate is 1:1.2 , the reaction temperature is -20±5°C.
[0086] Oxidation process: the organic base is triethylamine, and the molar ratio of dimethyl sulfoxide and organic base is 1:1.2.
[0087] Amination process: the temperature is between 40±5°C, acetic acid is replaced by benzoic acid solution, the concentration of benzoic acid solution is 20%, and the molar ratio of compound (formula IV) to benzoic acid is 1:1.2.
[0088] The emamectin benzoate product can be obtained at 147.82kg / h, with a content of 78.1%, a mass yield of 88.8%, and a purity of 85.2%.
Embodiment 3
[0090] A kind of synthetic method of emamectin benzoate, comprises the following steps:
[0091] The difference from Example 1 is that,
[0092] Protection process: the organic base is triethylamine, and the molar ratio of avermectin and triethylamine is 1:0.8.
[0093] Oxidation process: the organic base is triethylamine, the molar ratio of dimethyl sulfoxide and organic base is 1:1.2, the activator is the dichloromethane solution of triphosgene, the concentration of triphosgene is 30%, the compound (formula III) and The molar ratio of triphosgene is 1:0.35, and the reaction temperature is 0±5°C.
[0094] Amination process: temperature is between 40±5°C, acetic acid is replaced by p-toluenesulfonic acid solution, the concentration of p-toluenesulfonic acid solution is 20%, compound (formula IV) and p-toluenesulfonic acid The molar ratio is 1:0.5, and the molar ratio of p-toluenesulfonic acid and methylamine is 1:4.5.
[0095] Deprotection process: the temperature is contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com