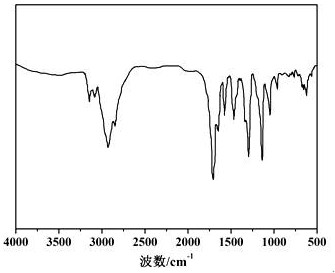
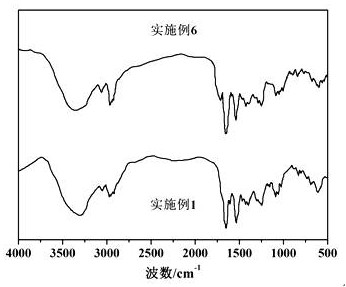
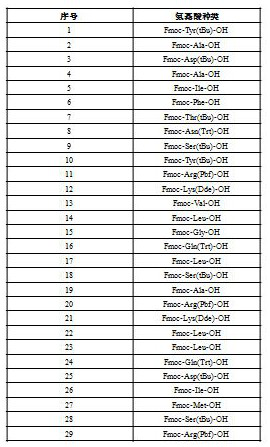
Method for preparing sermorelin acetate
A technology for the synthesis of acetic acid and peptides, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of increasing the difficulty of purification, increasing the cost of the final product, etc., to achieve the synergy of catalytic condensation performance, and enhance the promotion of non-specific immunity. Functional ability, high application value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of ionic liquid:
[0067] Add imidazole and NaH into THF, stir at 0°C for 45 min, return to room temperature, add 1-bromooctane, and stir for 24 h; extract with THF and purify by thin-layer silica gel column to obtain product A; among them, imidazole The molar ratio of NaH to NaH is 1:1; the solid-liquid ratio of imidazole to THF is 0.35 g / mL; the molar ratio of 1-bromooctane to imidazole is 1.1:1;
[0068] Dissolve 1,6-dibromohexane in acetonitrile at a concentration of 1.2 g / mL, heat to reflux, and slowly add the acetonitrile solution of product A (the molar ratio of 1,6-dibromohexane is 1:15.5) dropwise (Concentration: 0.16 g / mL), the system was reacted at 92°C for 24 h; the solvent was distilled off under reduced pressure, and washed 4 times with anhydrous ether to obtain product B;
[0069] Get product B and 4-(azidine-3-yl) piperazine-1-carboxylate tert-butyl ester (the molar ratio of both is 1: 1.3) and potassium carbonate is added (the molar ratio w...
Embodiment 2
[0098] The preparation of the ionic liquid was the same as in Example 1.
[0099] The difference between a method for preparing sermorelin acetate and Example 1 is that HBTU is used instead of HOBt in step 6;
[0100] NMM is used instead of DIC.
Embodiment 3
[0102] The preparation of the ionic liquid was the same as in Example 1.
[0103] The difference between a method for preparing sermorelin acetate and Example 1 is:
[0104]In step 2, the mol ratio of ionic liquid and DMAP is 1: 2;
[0105] In step 6, the molar ratio of ionic liquid to HOBt is 1:2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com