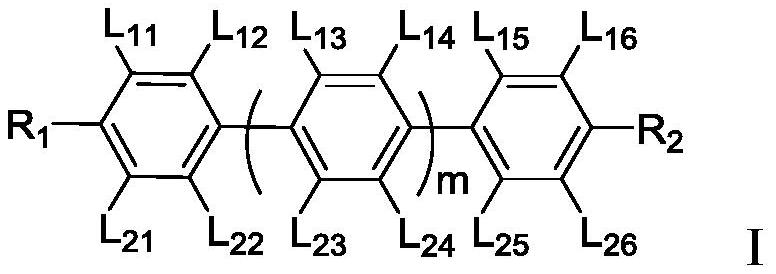
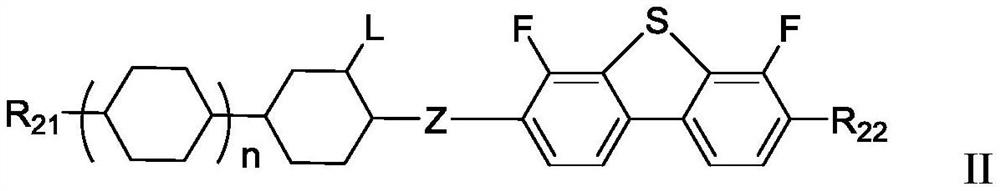
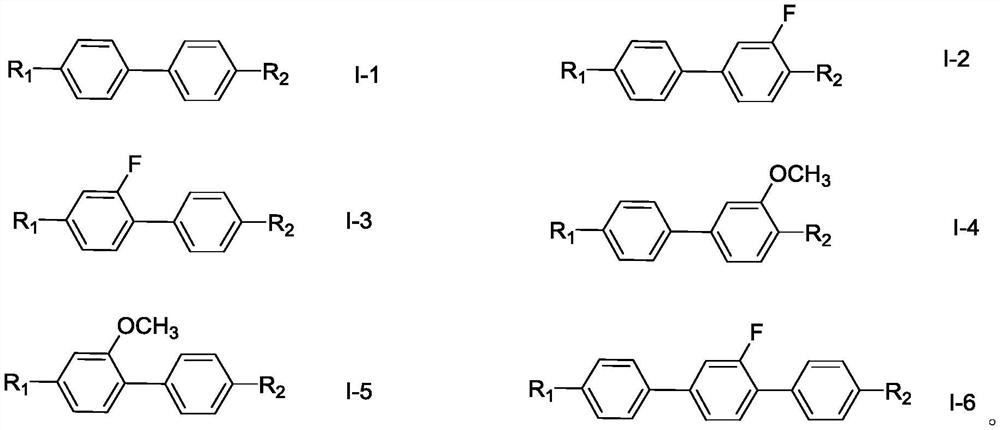
Polymerizable liquid crystal composition and application thereof
A technology for polymerizing liquid crystals and polymer compounds, used in negative liquid crystal compositions and their applications, polymerizable liquid crystal compositions and their application fields, can solve the problems of difficult complete reaction of RM, poor display, easy afterimages, etc., and achieve low RM residue, moderate dielectric anisotropy, good low temperature mutual solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] The synthesis of compound LDT-1, its specific preparation process is as follows:
[0091]
[0092] (1) Synthesis of LDT-1-2
[0093] Add 150ml of THF to a 500ml reaction flask, under the protection of nitrogen, add 23.8g (0.1mol) and 150ml of tetrahydrofuran to the reaction flask, and add 0.15mol of n-butyllithium n-hexane solution dropwise at -70~-80℃ under temperature control. Temperature controlled reaction for 1 hour, 28.2 g of triisopropyl borate was added dropwise at temperature controlled at -60~-70 °C, kept at temperature for 1 h, and then returned to -20 °C naturally. Add 400ml of 2M hydrochloric acid aqueous solution for acidification, perform conventional post-treatment, and recrystallize petroleum ether to obtain light yellow solid compound LDT-1-1, weight 24g, HPLC: 99.8%, yield: 85%.
[0094] (2) Synthesis of LDT-1-4
[0095] Add 28g of LDT-1-2, 100ml of DMF, 50ml of water, 27.6g of anhydrous potassium carbonate, LDT-1-336g, and 0.5g of palladium tetr...
Embodiment 2
[0104] The synthesis of LDT-2, its specific preparation process is as follows:
[0105]
[0106] (1) Synthesis of LDT-2-2
[0107] Add 150ml of THF to a 500ml reaction flask, under the protection of nitrogen, add 28.2g, 150ml of tetrahydrofuran to the reaction flask, add 0.15mol of n-butyllithium n-hexane solution dropwise at -70~-80°C under temperature control, and complete the temperature control reaction 1 28.2 g of triisopropyl borate was added dropwise at -60 to -70°C under temperature control, kept for 1 hour, and then returned to -20°C naturally. Add 400ml of 2M hydrochloric acid aqueous solution for acidification, perform conventional post-treatment, and recrystallize petroleum ether to obtain light yellow solid compound LDT-1-1, weight 27.1g, HPLC: 99.8%, yield: 83%.
[0108] (2) Synthesis of LDT-2-3
[0109] Add 32.6g of LDT-2-2, 100ml of DMF, 50ml of water, 27.6g of anhydrous potassium carbonate, 36g of LDT-1-3, 0.5g of palladium tetrakistriphenylphosphine into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com