Pharmaceutical composition as well as therapeutic agent and application thereof
A technology of composition and therapeutic agent, which is applied in the field of biopharmaceuticals, can solve the problems of increased survival rate of fat transplantation, achieve good rejection effect, improve facial atrophy, and increase the effect of fat survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The collection of embodiment 1 sample
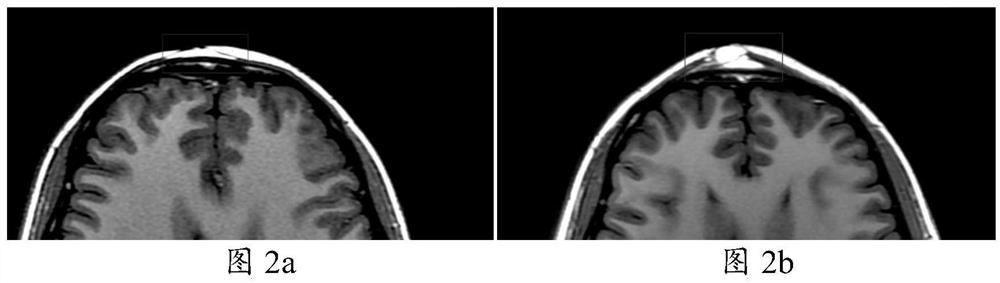
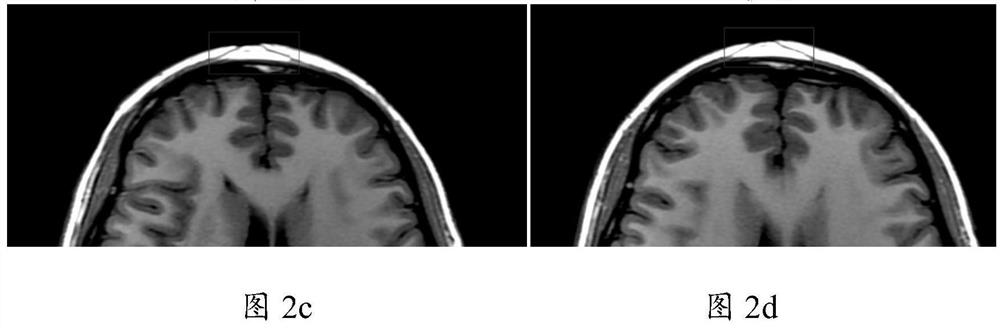
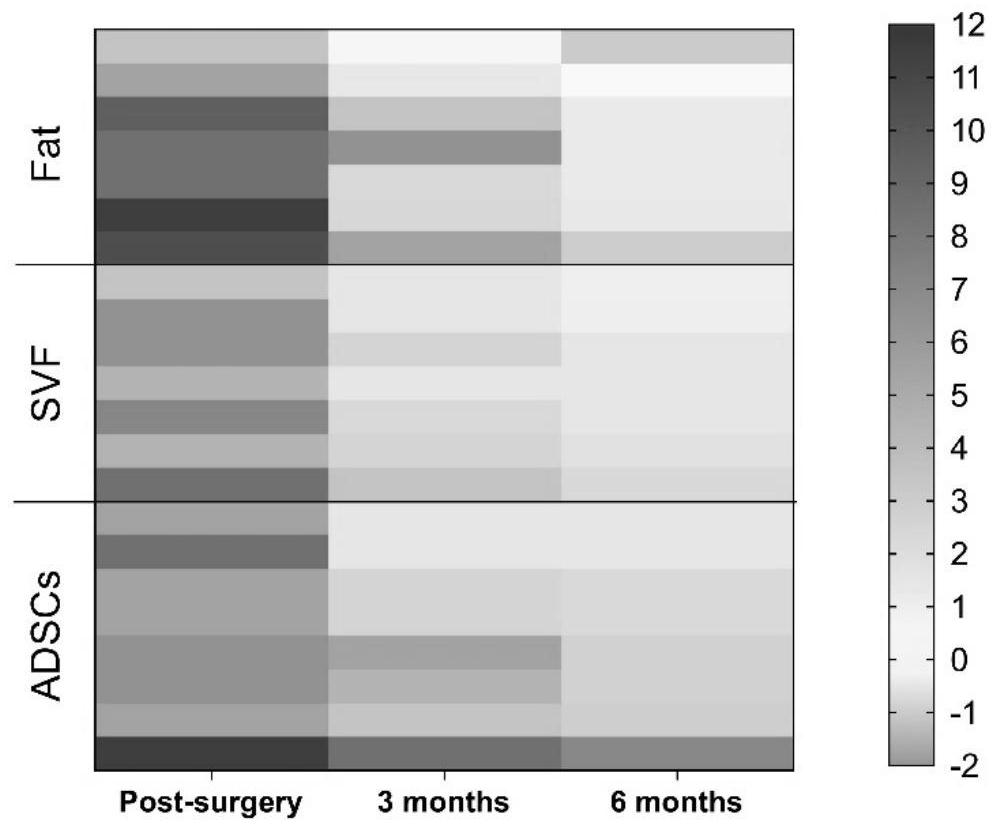
[0045] In this study, 22 patients (female: 15; male: 7) with facial depression caused by scleroderma were selected, 8 patients received ADSCs-assisted fat grafting (experimental group), and 7 patients received vascular stromal component (SVF)-assisted fat grafting. (standard control group) and 7 cases of simple fat transplantation group (blank control group). The study followed the statement of the Helsinki Guidelines, which was approved by the Institutional Ethics Committee (JMLL20180902) and registered with the Chinese Clinical Trials Registry (ChiCTR1900025717).
[0046] Sample inclusion criteria:
[0047] (1) Patients with localized scleroderma diagnosed by dermatology;
[0048] (2) The localized scleroderma is in a stable stage;
[0049] (3) Facial depression deformity caused by localized scleroderma;
[0050] (4) Age 18-50;
[0051] (5) The subject or legal representative gave informed consent, volunteered to be tested,...
Embodiment 2
[0060] The collection of embodiment 2 granular fat
[0061] Adipose tissue comes from healthy volunteers. Before collection, the age and sex of the donors are recorded in detail, and their health status is known through routine physical examination. Serological tests for HBV, HCV, HIV and syphilis are required to be negative. On the morning of the day of collection, no food or water was fasted, and all biochemical items and routine blood tests were checked before entering the operating room.
[0062] The liposuction area is generally located in the abdomen, anesthetized with tumescent fluid, formula of tumescent fluid: 1000mL normal saline + 2% lidocaine 30mL + 1:1000 epinephrine 1mL + 5% sodium bicarbonate 10mL. Use a 20mL syringe to connect a small-caliber sharp suction needle (diameter mm), draw it back after entering the subcutaneous area, and form a negative pressure, reciprocating suction in a spoke or fan shape. After the suction is completed, the syringe is still inve...
Embodiment 3
[0064] Example 3 Isolation and cultivation of adipose-derived stem cells
[0065] 1. Isolation of somatic cells
[0066] (1) Add an equal volume of normal saline to wash the fat three times: transfer the upper layer of fat to 50ml sterile centrifuge tubes, about 20ml per tube, add saline to 40ml per tube, centrifuge at room temperature in a horizontal centrifuge, 500 rpm, 5 minutes .
[0067] (2) Add 10ml of collagenase digestion solution to each tube, supplement with saline to 35ml per tube, shake and digest at 37°C for 15-35 minutes, until the fat is digested into a uniform turbid liquid, and no obvious lumps are visible.
[0068] (3) Use a 100 μm filter to filter into a 50ml sterile centrifuge tube, and rinse the original centrifuge tube and filter with normal saline. Use DPBS or normal saline to make up the filtered liquid to 40ml per tube, and centrifuge in a horizontal centrifuge at room temperature at 1500 rpm for 10 minutes.
[0069] (4) Suspend the cells with the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com