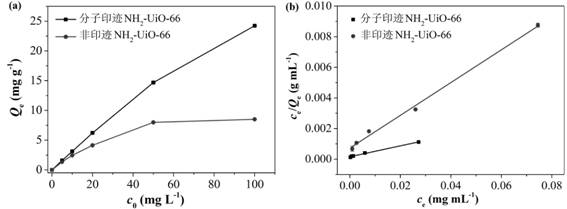
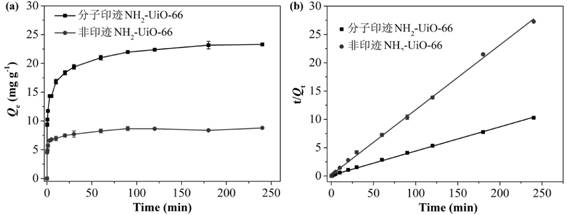
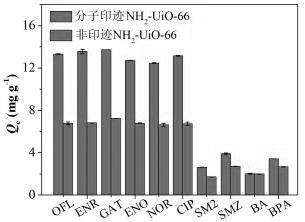
Molecularly imprinted material for efficiently adsorbing and removing fluoroquinolone antibiotics as well as preparation method and application of molecularly imprinted material
A technology of fluoroquinolones and molecular imprinting, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problems of low adsorption capacity, limit the application of MIPs, poor recognition effect, etc., and achieve improved adsorption capacity , Improve recognition efficiency, improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A Molecularly Imprinted NH for Efficient Adsorption and Removal of Fluoroquinolone Antibiotics 2 -The preparation method of UiO-66 material, comprises the steps:
[0044] (1) Weigh 240mg of zirconium tetrachloride and 78mg of pipemidic acid in a 100mL Erlenmeyer flask, and add 60mL of N'N-dimethylformamide (DMF) into it, dissolve it by ultrasonic, and stir to make it pre- Reaction 1h, obtain A solution; Wherein, the mol ratio of zirconium tetrachloride and pipemidic acid is 4: 1;
[0045] (2) Add 94 mg of 2-aminoterephthalic acid to solution A, dissolve it by ultrasonic, and stir for 30 minutes to obtain solution B;
[0046] (3) Transfer the B solution to a 100mL polytetrafluoroethylene lined bottle, then transfer it to a 100mL reaction kettle, place it in a blast drying oven, react at 120°C for 48h, cool it down to room temperature naturally, and take the reaction solution Centrifuge at 1000rpm for 15min, and discard the supernatant to obtain the intermediate product...
Embodiment 2
[0049] A Molecularly Imprinted NH for Efficient Adsorption and Removal of Fluoroquinolone Antibiotics 2 -The content of the preparation method of the UiO-66 material is basically the same as that of Example 1, except that the amount of pipemidic acid described in step (1) is 124.8 mg, wherein zirconium tetrachloride and pipemidic acid The molar ratio is 5:2.
Embodiment 3
[0051] A Molecularly Imprinted NH for Efficient Adsorption and Removal of Fluoroquinolone Antibiotics 2 -The content of the preparation method of the UiO-66 material is basically the same as that of Example 1, except that the amount of pipemidic acid described in step (1) is 156 mg, wherein the amount of zirconium tetrachloride and pipemidic acid The molar ratio is 2:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Equilibrium adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com