Pharmaceutical composition of imatinib salt for injection and preparation method thereof
A technology of imatinib and imatinib mesylate, applied in the field of medicine, can solve the problems of patients being unable to take oral administration, limiting the onset speed of imatinib, etc., so as to achieve good reproducibility of the preparation method and avoid long-term effects. Timed injection or multiple injections for highly controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
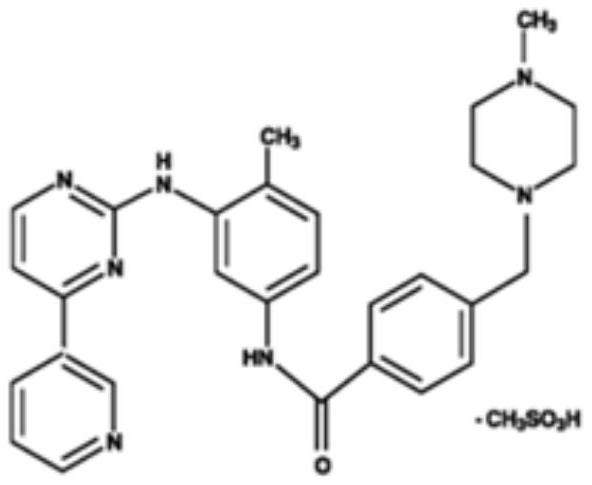
[0041] Add imatinib free base (5.0g, 10mmol) and methanesulfonic acid (1.06g, 11mmol) into ethanol (50ml) respectively to obtain a mixture, heat the mixture to 60°C, stir for 1h, filter the reaction solution hot, and cool down After stirring at room temperature for 2 h, the product was filtered and dried to obtain imatinib mesylate (5.19 g, yield 88%) as a white solid powder. Other salt forms were prepared in a similar manner.
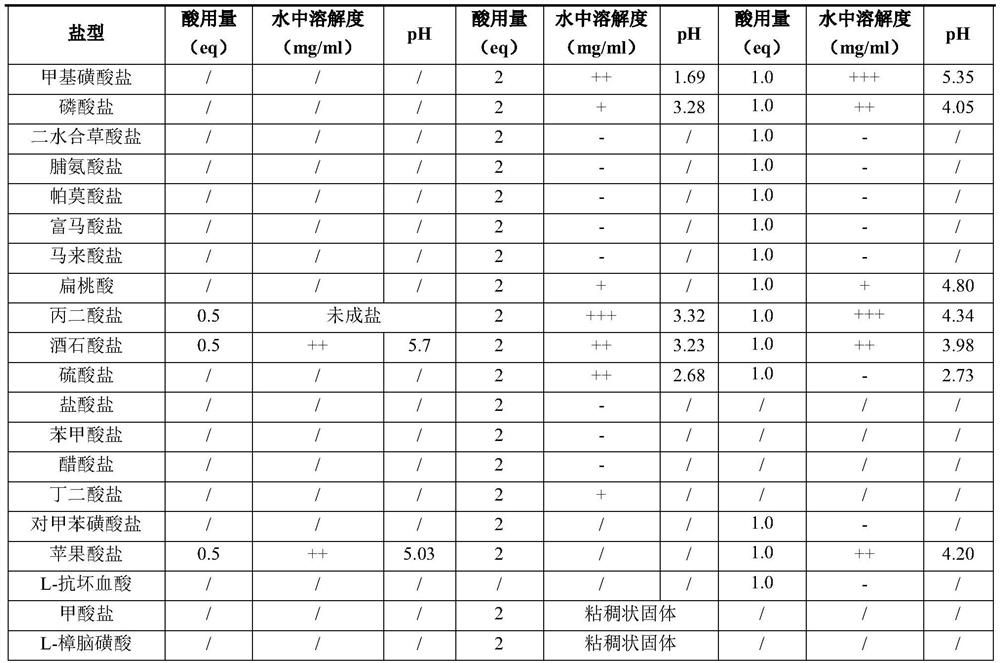
[0042] The properties, solubility and pH value of imatinib salt were tested respectively, and the results are shown in Table 1:
[0043] Table 1
[0044]
[0045]
[0046]- indicates a solubility of less than 25 mg / ml, + indicates a solubility of 25-50 mg / ml, ++ indicates a solubility of 50-100 mg / ml, +++ indicates a solubility of more than 100 mg / ml.
[0047] Solubility test method: Take imatinib salt, add a certain amount of purified water, shake or ultrasonically check whether it is dissolved or not, if it is not dissolved, continue to incre...
Embodiment 2
[0052] The salt of the present invention was dissolved in 0.9% sodium chloride aqueous solution, 5% glucose aqueous solution and 5% mannitol aqueous solution respectively, and the solubility at different temperatures was tested as shown in Table 2.
[0053] Table 2
[0054]
[0055]
[0056] - indicates a solubility of less than 25 mg / ml, + indicates a solubility of 25-50 mg / ml, ++ indicates a solubility of 50-100 mg / ml, +++ indicates a solubility of more than 100 mg / ml.
[0057] The result shows: in 0.9% sodium chloride solution, the solubility of most of the salts is low, there are different degrees of precipitation, and the storage stability is poor; although the solubility of tartrate in 0.9% sodium chloride aqueous solution is still 50~100mg / ml, but with the decrease of tartrate concentration, precipitation will occur. The pharmaceutical composition of the present invention is stable in storage and not easy to separate out.
Embodiment 3
[0059] Preparation of imatinib tartrate injection composition
[0060]
[0061] Preparation method: According to the prescription, add imatinib tartrate and additives into 60-70ml of water, stir until completely dissolved, and then dilute to 100ml with water. After being filtered through a 0.22 μm microporous membrane, the mixture was divided into vials to obtain the imatinib tartrate injection composition. The composition can also be freeze-dried to obtain a freeze-dried powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com