Nasal gel agent containing risperidone and preparation method of nasal gel agent
A risperidone nasal and gel technology, applied in the field of pharmaceutical preparations, can solve problems such as unsatisfactory curative effect, unfavorable industrial production, low bioavailability, etc. effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
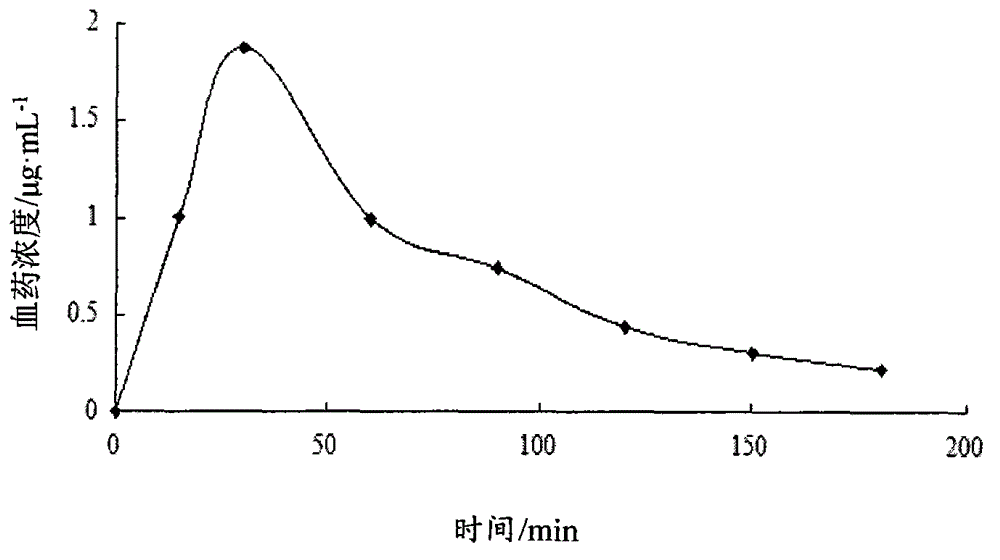
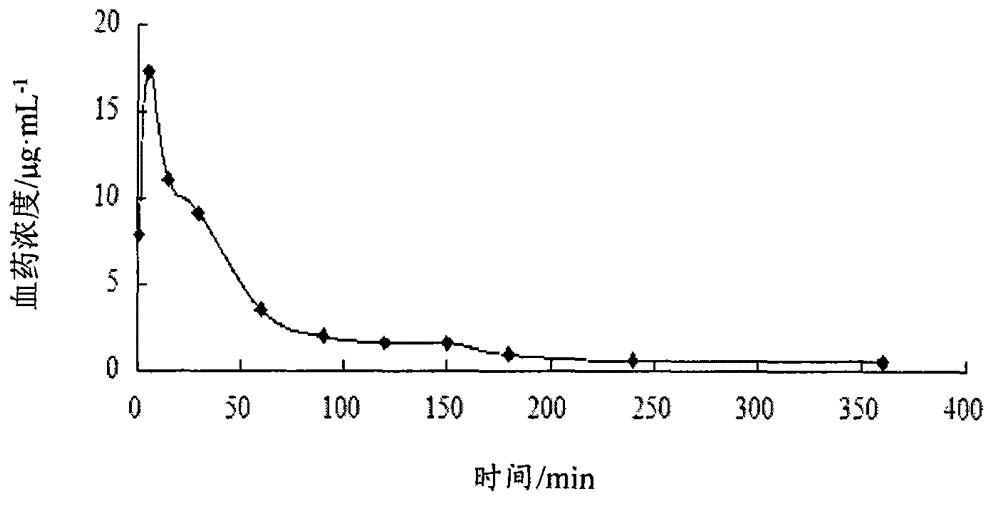
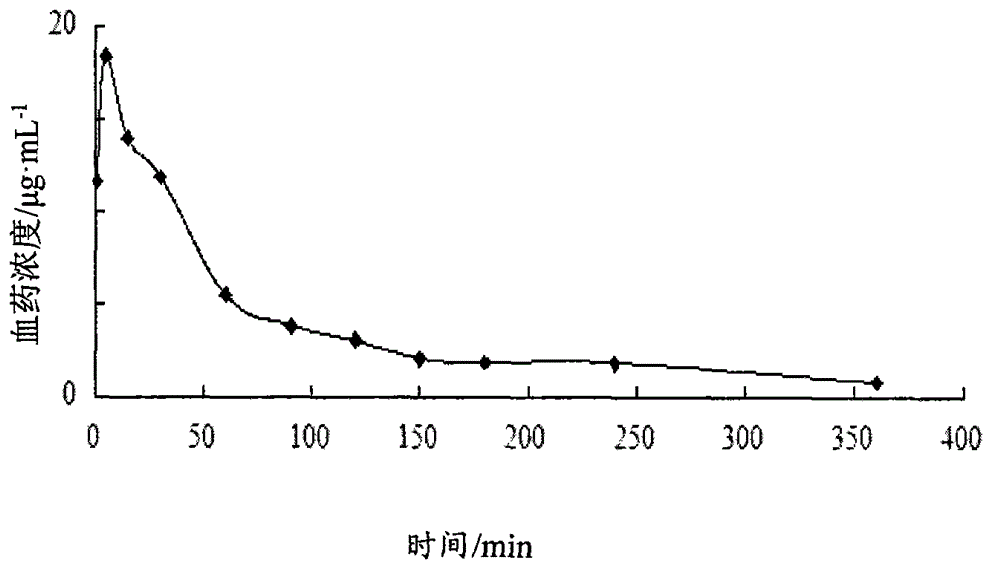
Image
Examples
Embodiment 1
[0034] Prescription 1: The following are all calculated by mass fraction
[0035]
[0036]
[0037] Preparation process: Weigh the prescribed amount of carbomer and sodium alginate, sprinkle them in an appropriate amount of purified water, and let them sit for 24 hours to fully swell, then adjust the pH to 6.0 with hydrochloric acid to obtain a blank gel matrix; separately take risperidone, azone, After ethylparaben is mixed with propylene glycol and kneaded evenly, add an appropriate amount of purified water, heat it in a water bath at 60°C to dissolve completely, add it to the blank gel matrix under constant stirring, knead it well, and finally add water to the full amount, That is, the drug nasal gel is obtained.
Embodiment 2
[0039] Prescription 2: The following are all calculated by mass fraction
[0040]
[0041] Preparation process: Weigh the prescribed amount of sodium carboxymethyl cellulose, sprinkle it in an appropriate amount of purified water, let it swell fully, heat it to dissolve, adjust the pH to 6.5 with sodium hydroxide, and obtain a blank gel matrix; take another dimethyl cellulose - After adding purified water to β-cyclodextrin to dissolve, then add glycerin, risperidone, benzalkonium bromide, and sodium chloride in sequence, heat in a water bath at 60°C to dissolve completely, and add it to the Grind thoroughly in the blank gel matrix, and finally add water to the full amount to obtain the nasal gel of the drug.
Embodiment 3
[0043] Prescription 3: The following are all calculated by mass fraction
[0044]
[0045] Preparation process: Weigh the prescribed amount of carbomer, sprinkle it in an appropriate amount of purified water, let it stand for 24 hours to fully swell, adjust the pH to 6.0 with triethanolamine, and obtain a blank gel matrix; separately take risperidone and Tween-80 to fully grind After uniformity, add purified water, heat in a water bath at 60°C to dissolve, then add chlorobutanol, sorbitol, and glucose to dissolve in sequence, and add them to the blank gel matrix under constant stirring, fully Grind well, and finally add water to the full amount to obtain the nasal gel of the drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com