Recombinant African swine fever virus antigen cocktail vaccine and application thereof
An African swine fever virus and cocktail technology, which is applied in the field of recombinant African swine fever virus antigen "cocktail" vaccine, can solve the problems of adverse reactions, numerous protein structures, and the inability of ASF inactivated vaccines to provide immune protection, and achieve easy purification and expression The effect of high dose and good immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
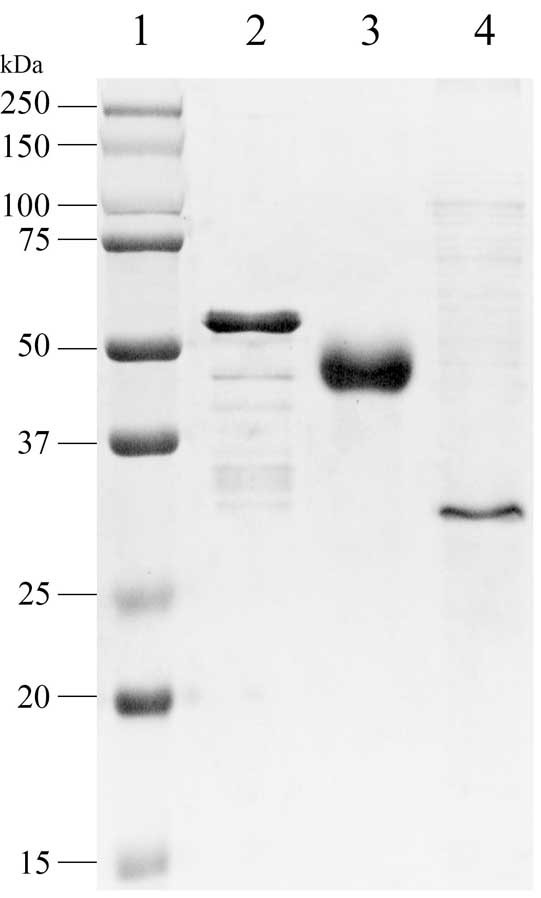
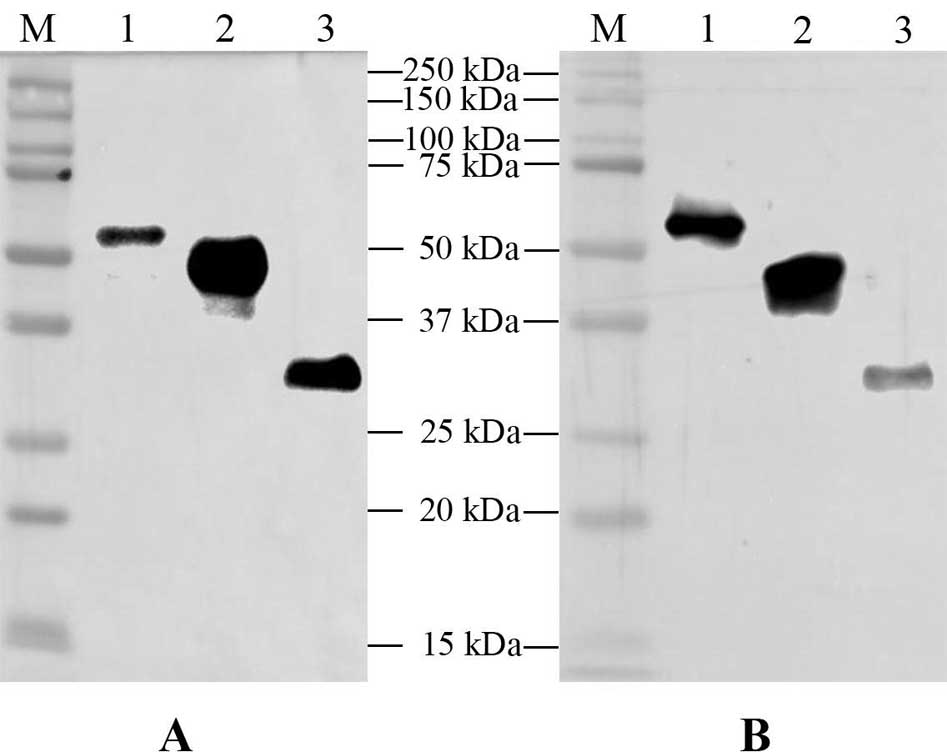
[0026] The inventors of the present invention found in their research that three recombinant ASFV antigen fusion proteins were obtained through gene fusion and tandem expression, namely p30-modified p54, p72 epitope-pE248R N-segment amino acid sequence and CD2v N-segment amino acid sequence- Amino acid sequence of segment C of pEP153R. After mixing these three recombinant proteins in a certain proportion, they are compatible with adjuvants to form a "cocktail" vaccine, which induces neutralizing antibodies and cellular immune responses after immunizing pigs, suggesting that this vaccine form is a promising ASF subunit vaccine . The three recombinant ASFV antigen fusion proteins are as follows:
[0027] (1) Recombinant ASFV antigen fusion protein PM, the recombinant protein is formed by fusion of ASFV p30 and modified p54 (mp54) through the linker (Linker), and the general formula of the recombinant protein is p30-(Linker) 3 -mp54;
[0028] (2) Recombinant ASFV antigen fusio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com