Application of polyesteramide compound in preparation of medicine for preventing and/or treating blood system diseases
A polyester amide and blood system technology, applied in the field of biomedicine, can solve the problems of single polymer function and inability to regulate the immune microenvironment of the blood system, and achieve safety assurance, low toxicity, and regulation of the immune microenvironment of the blood system Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
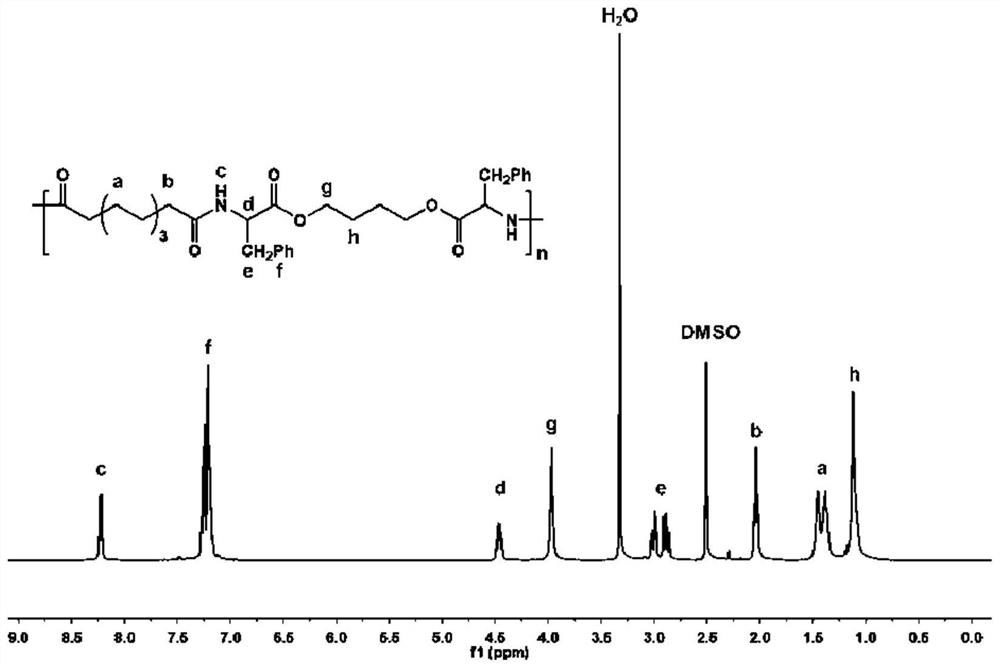
[0082] The synthesis of embodiment 1 polyester amides compound (8P4)
[0083] (1) Synthetic monomer p-nitrophenol sebacate (N8)
[0084] Dissolve p-nitrophenol (0.31 mol) and triethylamine (0.32 mol) in 500 mL of pyruvic acid together and cool in ice water at 0°C to obtain solution a. Dissolve sebacoyl chloride (0.15mol) in 100mL of pyruvic acid to obtain solution b, then add solution b dropwise to solution a, stir in an ice-water bath at 0°C for 2 hours, and gradually heat the reaction to room temperature under stirring for 12 hours before pouring Pour it into 2000mL distilled water to precipitate the product, filter it with suction, wash the precipitate, recrystallize it three times with ethyl acetate, dry it in vacuum at 40°C to constant weight, and obtain monomer N8, which is sealed and stored. Dissolve the purified monomer N8 in deuterated DMSO by 1 H NMR identified its chemical structure. Its chemical structural formula is as follows:
[0085]
[0086] (2) Synthes...
Embodiment 2
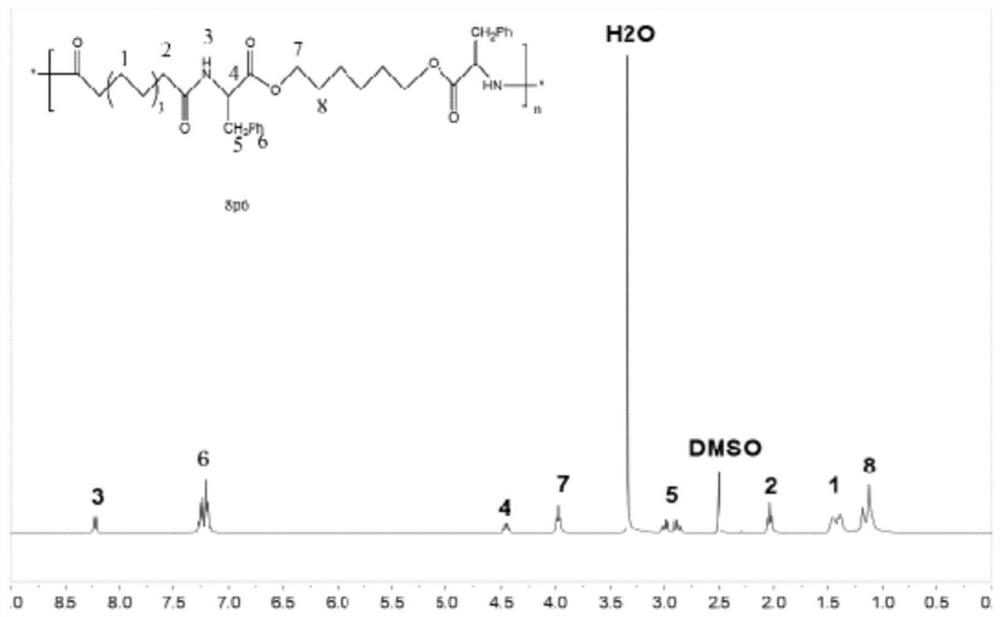
[0093]The synthesis of embodiment 2 polyester amides compound (8P6)
[0094] (1) Synthetic monomer N8
[0095] Consistent with the method of Example 1.
[0096] (2) Synthesis of monomer phenylalanine-6 (Phe-6)
[0097] Mix L-phenylalanine (0.04mol) and 1,6-hexanediol (0.02mol) directly, then add toluene (400mL) and p-toluenesulfonic acid monohydrate (0.082mol) and heat to 130°C, stir Reflux for 24 hours, produce 2.16mL (0.12mol) of water, cool to room temperature, pour into toluene to precipitate, filter, dissolve the filter residue in isopropanol at 75°C under stirring, then cool to 4°C to precipitate, repeat 3 times , to obtain monomeric Phe-6, sealed and preserved. Dissolve the purified monomeric Phe-6 in deuterated DMSO by 1 H NMR identified its chemical structure. Its chemical structural formula is as follows:
[0098]
[0099] (3) Synthesis of polyester amide compounds (8P6)
[0100] Dissolve monomer N8 (5 mmol) and monomer Phe-6 (5 mmol) in anhydrous DMSO (8...
Embodiment 3
[0104] Embodiment 3: the preparation of the polyester amide compound nanoparticle of loading doxorubicin
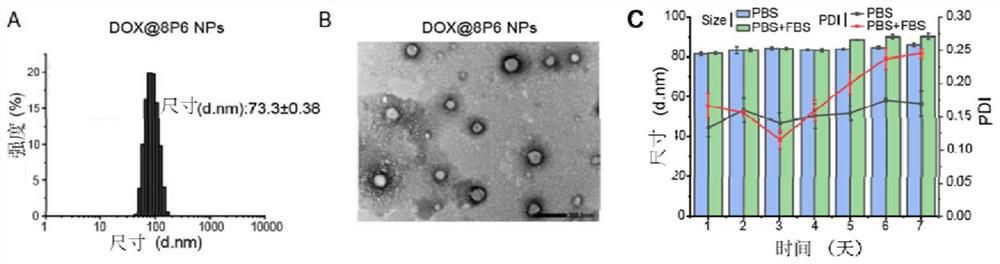
[0105] Dissolve 3.6 mg of 8P6 prepared in Example 2, 2 mg of DSPE PEG 2000 and 0.4 mg of doxorubicin (DOX) in 0.6 mL of dimethyl sulfoxide, and add dropwise to 6 mL of deionized water under magnetic stirring (1000 rpm) to form Doxorubicin-loaded polyester amide nanoparticles (DOX@8P6 NPs), and then the organic solvent DMSO in the medium was removed by multiple ultrafiltration. The prepared nanoparticles were stored in a refrigerator at 4°C until use.
[0106] Characterization tests:
[0107] The DOX@8P6 NPs were subjected to DLS and TEM tests. like image 3 Shown in A: The average particle size of DOX@8P6 NPs is about 73nm, and the particle size is small, which is conducive to the uptake of the carrier by tumor cells and exerts its anticancer effect. like image 3 Shown in B: The TEM image of DOX@8P6NPs shows that the nanoparticles are spherical.
[0108] The stabil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com