Preparation method of acemetacin impurity D
A technology of acemetacin and impurities, which is applied in the field of drug impurity synthesis, can solve the problems of no acemetacin impurity D, etc., and achieve the effect of shortening linear steps, short linear steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
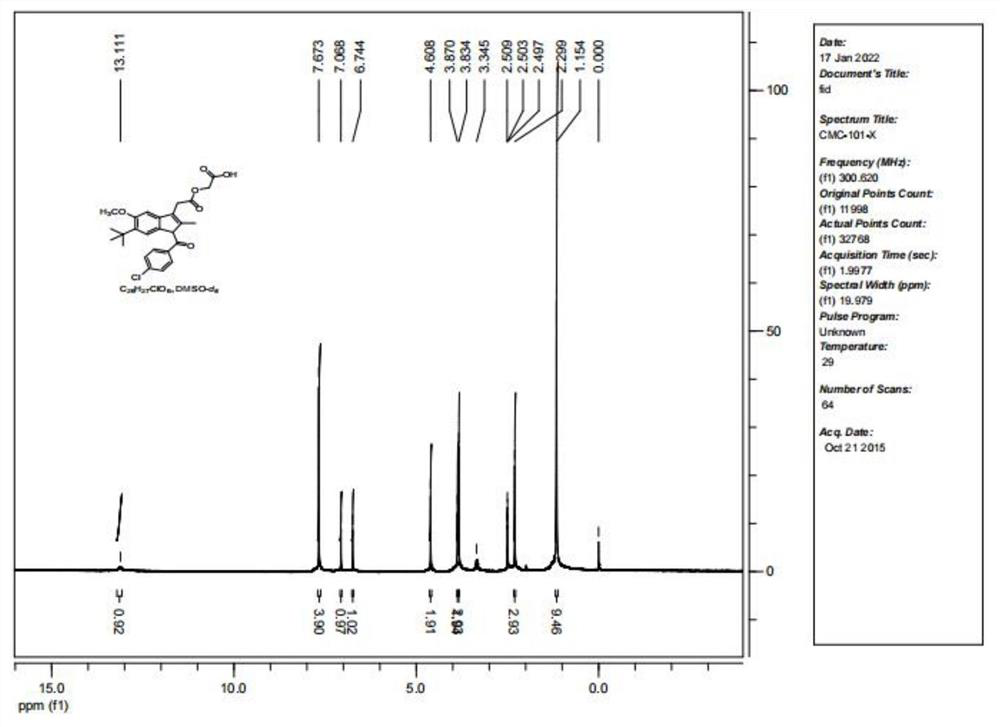
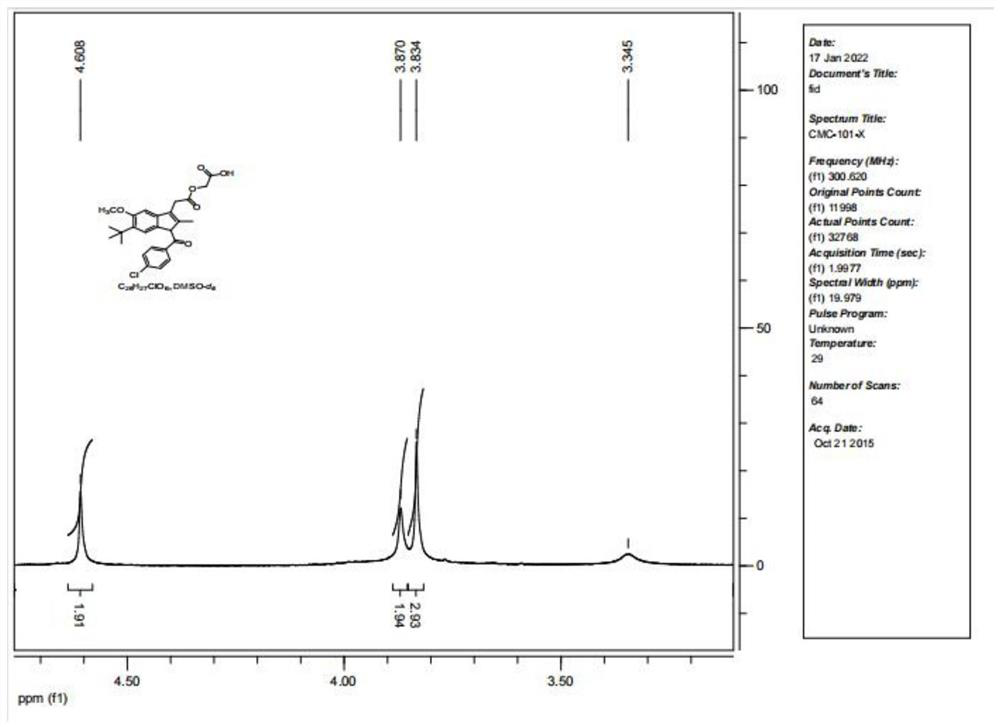
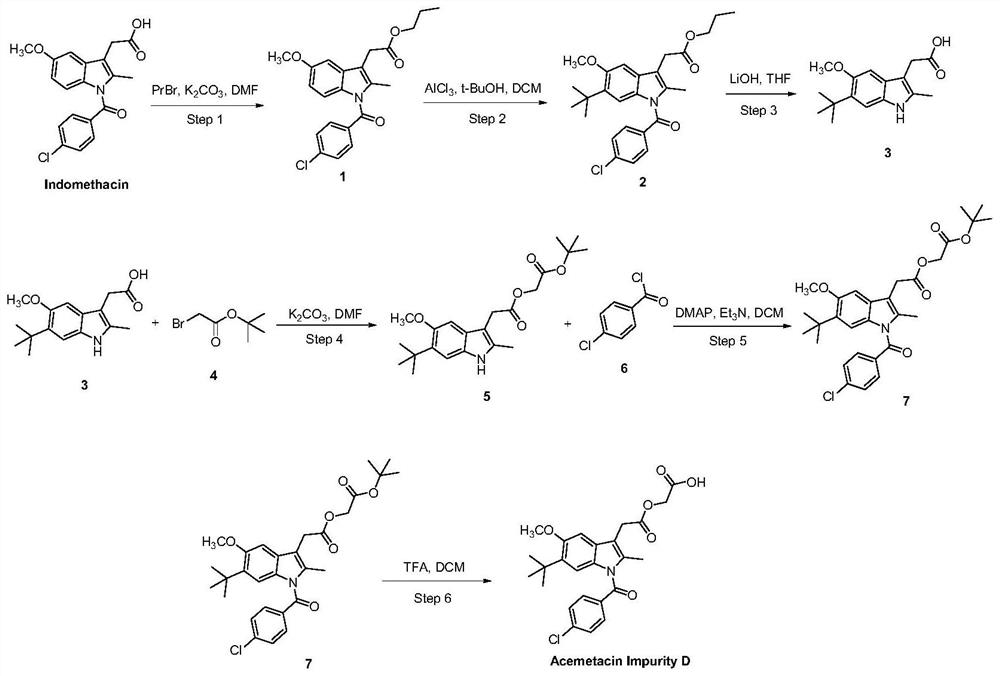
[0039] A method for rapidly preparing acemetacin impurity D, using indomethacin as a raw material, and preparing acemetacin impurity D through 6 steps of reaction, the synthetic route is as follows figure 2 Shown, where PrBr (1-bromopropane), DMF (N, N-dimethylformamide), DMAP (4-dimethylaminopyridine).
[0040] The specific preparation method is as follows:
[0041] (1) Preparation of indomethacin propyl ester (intermediate 1):
[0042] In a 500ml three-necked bottle, add indomethacin 10.0g (28mmol), potassium carbonate (K 2 CO 3 ) 8.31g (58.2mmol), 200mL of solvent DMF, stirred evenly, then added 1.1 times the equivalent of 1-bromopropane 3.82g (31.3mmol), stirred and reacted at room temperature for 12h, TLC monitored that the reaction was complete, and there was an insoluble precipitate in the system, Remove the organic solvent DMF under reduced pressure, add 200mL of water, extract twice with 150ml ethyl acetate respectively, combine the organic phases, wash the organi...
Embodiment 2
[0063] Other contents are as in Example 1, wherein in step (1) indomethacin and 1.5 times equivalent of 1-bromopropane are used as raw materials, in the solvent DMF, under the condition of alkaline pH 12, the reaction is stirred at room temperature for 12 hours, and the preparation 3 -(2-propyl acetate) substituted indole intermediate product 1;
[0064] (2) Dissolve the intermediate 1 prepared in the previous step in the substrate, that is, DCM that is 15 times the mass of intermediate 1, add an organic alcohol, cool the mixture to 0°C, and then add the substrate, that is, intermediate 1 4-5 times the equivalent of the catalyst, the mixture was stirred, and reacted at 40°C for 18 hours, and the mixture was separated and purified by extraction to obtain a brown intermediate compound 2;
[0065] (3) Intermediate 2 with a tert-butyl group introduced at the 6-position was added to a THF aqueous solution, and the substrate, that is, 3 times the equivalent of LiOH of Intermediate 2...
Embodiment 3
[0069] Other contents are as in Example 1, wherein in step (1) indomethacin and 1.3.5 times the equivalent of 1-bromopropane are used as raw materials, in the solvent DMF, under the condition of alkaline pH 12, the reaction is stirred at room temperature for 12h, Preparation of 3-(2-propyl acetate) substituted indole intermediate product 1;
[0070] (2) Dissolve the intermediate 1 prepared in the previous step in the substrate, that is, DCM that is 14 times the mass of intermediate 1, add an organic alcohol, cool the mixture to 0°C, and then add the substrate, that is, intermediate 1 4-5 times the equivalent of the catalyst, the mixture was stirred, and reacted at 40°C for 17h, and the mixture was separated and purified by extraction to obtain a brown intermediate compound 2;
[0071] (3) Intermediate 2 with a tert-butyl group introduced at the 6-position was added to THF aqueous solution, that is, LiOH, which was 3 times the equivalent of Intermediate 2, was stirred and react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com