Hydroxamic acid compound and application thereof
A compound and application technology, applied in the field of medicinal chemistry, can solve the problems of normal tissue cell toxicity and side effects, easy drug resistance, lack of HDAC subtype selectivity, etc., achieve good anticancer activity, high yield, and easy post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] Preparation of intermediates
[0063] Intermediate: Preparation of methyl 2-(2,4-dimethoxyphenyl)thiazole-5-carboxylate
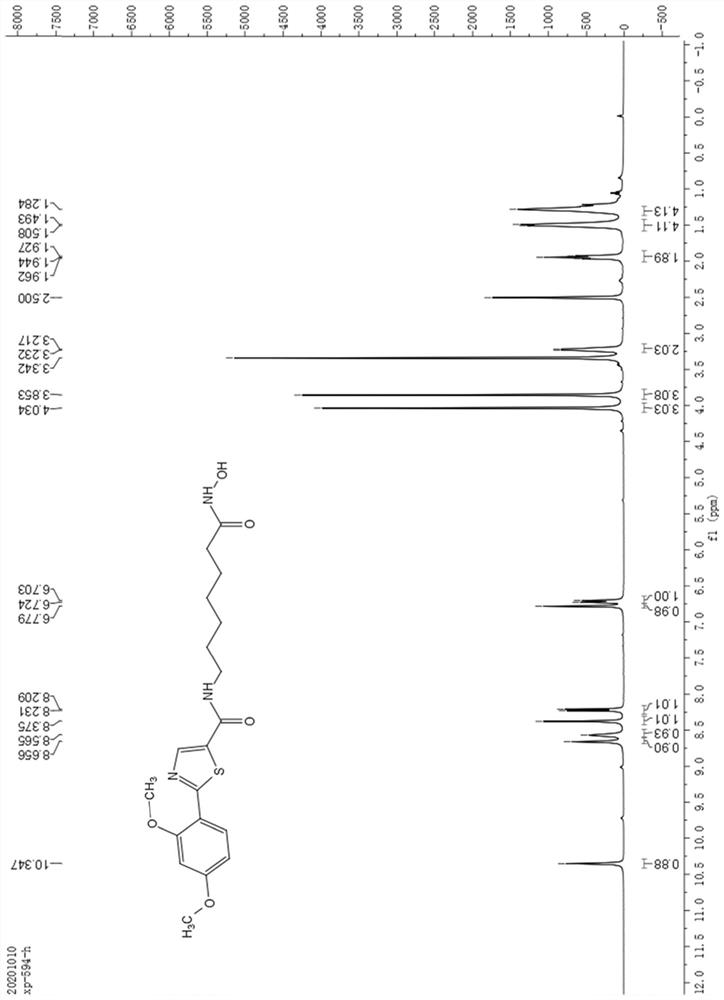
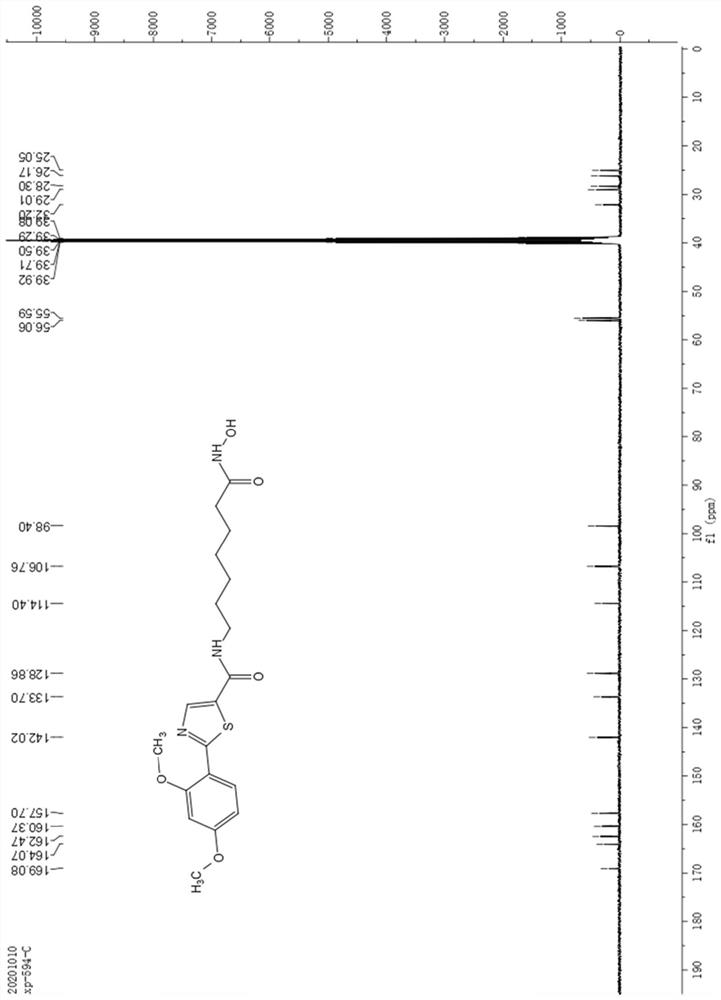
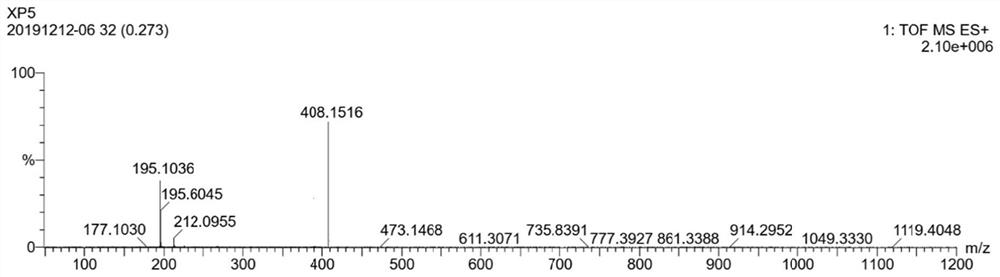
[0064] Dissolve 0.2g of 2-bromo-5-carboxylate thiazole in 10mL of toluene, add 0.2g of 2,4-dimethoxyphenylboronic acid, then add 0.11g of tetrakistriphenylphosphine palladium and 0.5g of potassium phosphate, in React at 90°C for 10 hours under a nitrogen atmosphere. After the reaction is complete as monitored by thin-layer chromatography, the reaction solution is extracted with ethyl acetate (20mL×3), allowed to stand for liquid separation, and the organic phase is washed with saturated brine (5mL×3) , then dried with anhydrous sodium sulfate, suction filtered, and removed ethyl acetate under reduced pressure to obtain a white solid, which was subjected to column chromatography with petroleum ether: ethyl acetate to obtain 0.218 g of white solid powder with a yield of 86%. The white solid powder obtained by column chromatography was identified by th...
Embodiment 3
[0091] Example 3 In vitro antitumor activity research
[0092] The in vitro antitumor activity of the compound of the present invention is proved by the following method test. These effects suggest that the compounds of the present invention are useful in the treatment of cancer. The specific test method is as follows:
[0093] 2-(2,4-dimethoxyphenyl)-N-(7-(hydroxylamino)-7-oxoheptyl)thiazole-5-carboxamide prepared by MTT method detection embodiment 1 and implementation In vitro antitumor activity of 2-(2,4-dimethoxyphenyl)-N-(7-(hydroxyamino)-7-oxoheptyl)imidazole-5-carboxamide prepared in Example 2. Tumor cell lines were purchased from ATCC in the United States, and the standard MTT method was used to determine the effect of the test compound on human colorectal cancer cell line HCT-116, human non-small cell lung cancer cell A549, human breast cancer cell MCF-7, melanoma cell B16-F10 and The anti-proliferation activity of gastric cancer cell line YCC resistant to HDAC inh...
Embodiment 4
[0098] Example 4 Research on Inhibition of HDAC in Vitro
[0099] The in vitro antitumor activity of the compounds VIII and IX of the present invention is demonstrated by the following method. These effects indicate that compounds VIII, IX of the present invention can be used to effectively inhibit HDAC6. The specific test method is as follows:
[0100] The enzyme inhibitory activity of compounds VIII and IX was determined by fluorescence analysis, wherein HDAC1 (#ab101661) and HDAC6 (#ab42632) enzymes were purchased from Abcam, HDAC3 (#BML-SE515-0050) was purchased from Enzo, HDAC8 (# H90-30H-05) was purchased from SignalChem. The buffer contains 25mmol / L Tris (pH 8.0), 1mmol / L MgCl 2, 0.1mg / mL BSA, 137mmol / L NaCl, 2.7mmol / L KCl, of which HDAC (HDAC1, 7.2ng / well; HDAC3, 3.4ng / well; HDAC6, 15ng / well; HDAC8, 22ng / well), total volume 40 μL. The compound to be tested (10 dilution concentrations, followed by 500μM, 125μM, 31.25μM, 7.81μM, 1.95μM, 0.49μM, 0.12μM, 0.03μM, 7.6nM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com