Application of NVP-BGJ398 in preparation of medicine for treating rMED
A technology of NVP-BGJ398 and drugs, applied in the field of biomedicine, can solve the problems of complex dysplasia mechanism and difficult clinical diagnosis and treatment, and achieve the effect of improving bending deformity and chondrocyte differentiation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
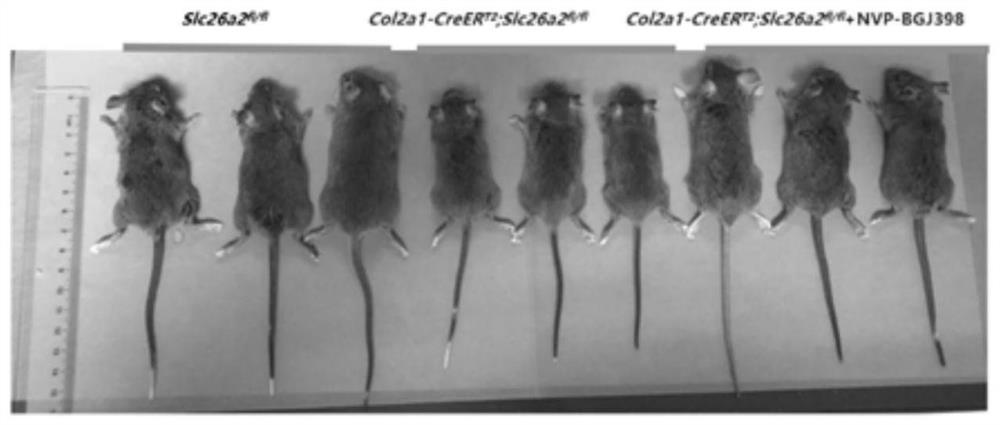
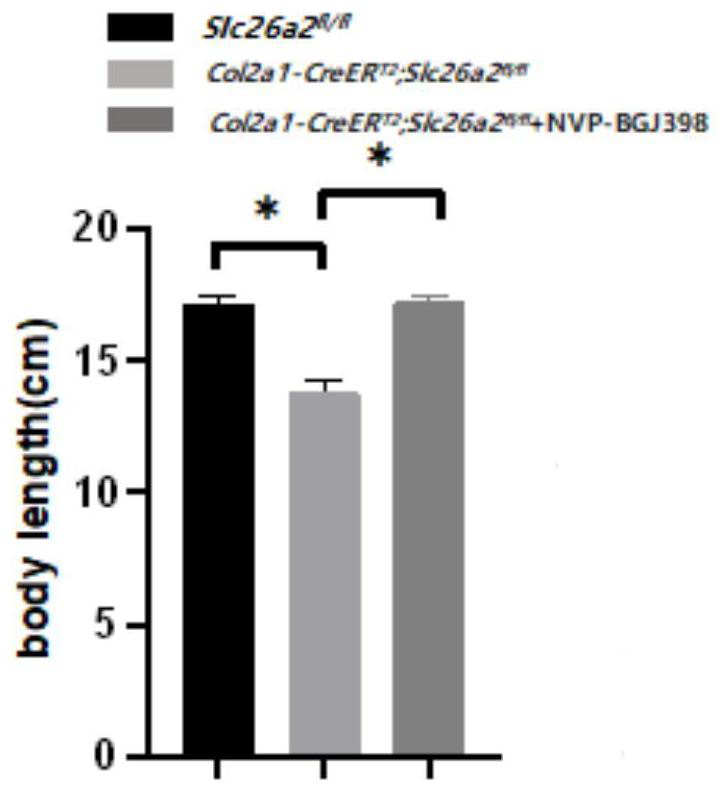
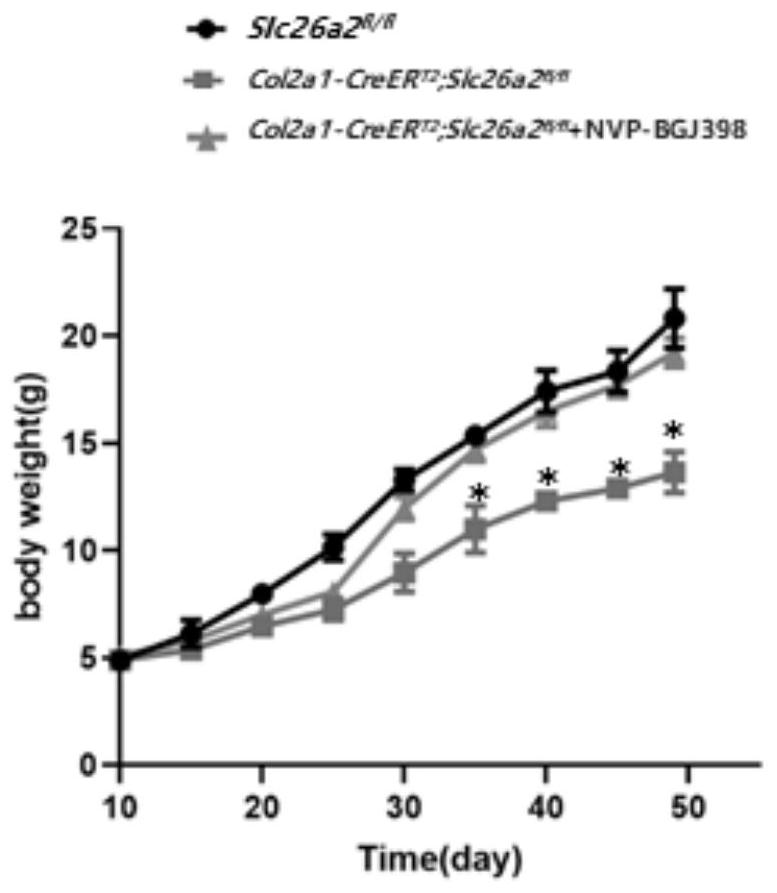
[0023] Establish the rMED phenotype induced by loss of SLC26A2 function, as well as the NVP-BGJ398 administration strategy and observe the gross morphology.
[0024] Using the cre-loxp system induced by Tamoxifen, a mouse model of Slc26a2-related chondrodysplasia was established, and the FGFR3 inhibitor NVP-BGJ398 was given to intervene. The experimental groups were: control group Slc26a2 fl / fl , experimental group Col2a1-CreER T2 ,Slc26a2 fl / fl , Col2a1-CreER T2 , Slc26a2 fl / fl +NVP-BGJ398. Tamoxifen was dissolved in 95% corn oil + 5% absolute ethanol to make a 50 mg / mL working solution, and NVP-BGJ398 was dissolved in 5% DMSO solution. The model of the induced dysplasia group was: intraperitoneal injection of Tamoxifen (100ug / day) 10 days after birth, continuous administration for 5 days, after an interval of 5 days, a cycle of intraperitoneal Tamoxifen-induced administration once a week, and the intervention until the 49th day After the sampling (n=4 ). Drug intervent...
Embodiment 2
[0029] [Example 2] Observation of chondrocyte proliferation and apoptosis by histological section staining
[0030] P49 tibial growth plate cartilage tissue section: Collect the tibial growth plate specimens of the mice used in this experiment, fix them in 4% PFA solution and keep at 4°C overnight; wash with PBS, place in 10% EDTA decalcification solution for 2 weeks, PBS Washed, dehydrated in 30% sucrose solution, embedded with OCT, and frozen into sections.
[0031]Detection of chondrocyte proliferation and apoptosis: Immunofluorescence detection of proliferative chondrocyte marker Ki67 and apoptotic chondrocyte marker Tunel was used to evaluate the improvement of drugs on chondrocyte proliferation and apoptosis. The frozen sections were taken out and placed at room temperature for 2 hours to return to room temperature, and the embedding agent was washed away with PBS. The immunohistochemical pen was used to outline the stained area, and the immunofluorescence penetrating s...
Embodiment 3
[0033] [Example 3] NVP-BGJ398 significantly improved collagen differentiation and FGFR3 signaling pathway activated by SLC26A2 loss of function in vivo
[0034] Chondrocyte FGFR3-specific marker detection: take out the frozen section and let it return to room temperature, and wash away the embedding agent with PBS. The stained area was outlined with an immunohistochemical pen, and the immunofluorescence penetrating solution was added dropwise to punch holes. After incubation at room temperature for 10 minutes, wash with PBS 3 times, 5 minutes each time. Sections were then treated with immunofluorescent blocking solution and incubated at room temperature for 10 minutes. Wash 3 times with PBS, 5 minutes each time. Dilute anti-p-STAT1 and anti-p-ERK1 / 2 primary antibodies at a ratio of 1:100 with immunofluorescence primary antibody diluent, and incubate overnight in a 4°C refrigerator. Wash 3 times with PBS for 5 minutes each time, then dilute the secondary antibody with immunof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com