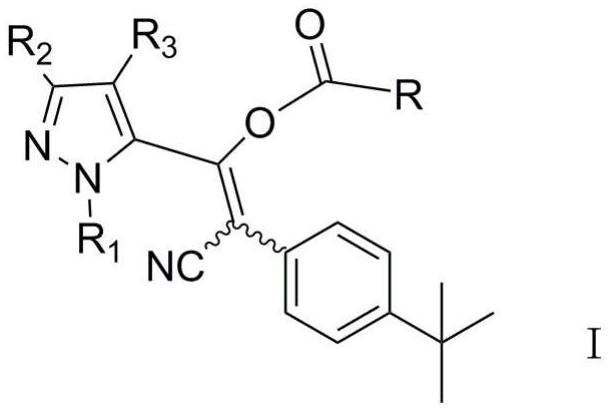
Pyrazolyl acrylonitrile compound as well as preparation method and application thereof
A technology of pyrazolyl acrylonitrile and compounds, applied in the field of pesticides, can solve the problems of agricultural production loss, loss of sensitivity to various types of pesticides, etc., and achieve the effect of high-efficiency acaricidal activity and safe preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 2-(4-tert-butylphenyl)-3-(1,3,4-trimethylpyrazol-5-yl)-3-(2-chloro-ethoxy)-formyloxyacrylonitrile Synthesis of (Z / E)
[0068] (1) Synthesis of intermediate crude product (2-(4-tert-butylphenyl)-3-(1,3,4-trimethylpyrazol-5-yl)-3-hydroxyacrylonitrile):
[0069] In a 250mL three-necked reaction flask equipped with a magnetic stirrer, thermometer, water separator and condenser, add 7.4g of ethyl 1,3,4-trimethylpyrazole-5-carboxylate, 7.6g of p-tert-butyl Phenylacetonitrile, 4mL ethylene glycol ethyl ether and 100mL n-heptane, stirred at room temperature for 0.5h, heated to 135°C for 1h, then added dropwise 12.4g of 30% sodium methoxide solution, and continued to reflux for 5h after the dropwise addition; The reaction solution was cooled to room temperature, poured into 250 mL of ice water, extracted three times with 100 mL of ethyl acetate, then adjusted the aqueous phase to pH=5 with 30% hydrochloric acid, extracted three times with 100 mL of ethyl acetate, and extracted ...
Embodiment 2~16
[0073] According to the preparation method of Example 1, the pyrazolylacrylonitrile compounds of Examples 2-16 were prepared by using the corresponding acid chlorides and the intermediate crude product prepared in Example 1.
[0074] Specifically: in Example 2, reacting phenyl chloroformate with the intermediate crude product to prepare Compound No.3 and No.4; in Example 3, reacting cyclohexanecarbonyl chloride with the intermediate crude product to prepare Compound No.5 and No.6 In embodiment 4, 4-ethynyl-benzoyl chloride is reacted with intermediate crude product to prepare compound No.7 and No.8; In embodiment 5, phenylpropyloyl chloride is reacted with intermediate crude product to prepare compound No.9 and No. .10; in embodiment 6, cyclohexa-3-ene-1-formyl chloride is reacted with intermediate crude product to prepare compound No.11 and No.12; in embodiment 7, cyclopentacarbonyl chloride is reacted with intermediate crude product to prepare compound No.13 and No.14; Compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com