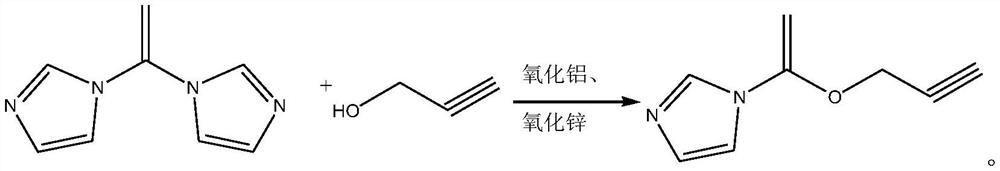
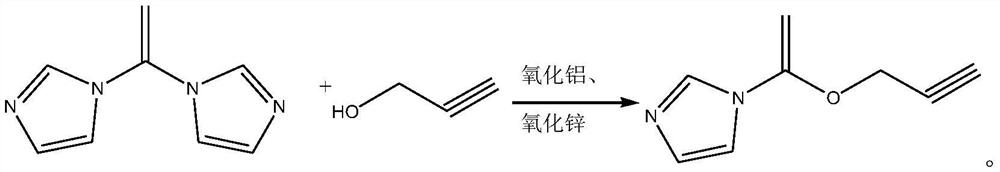
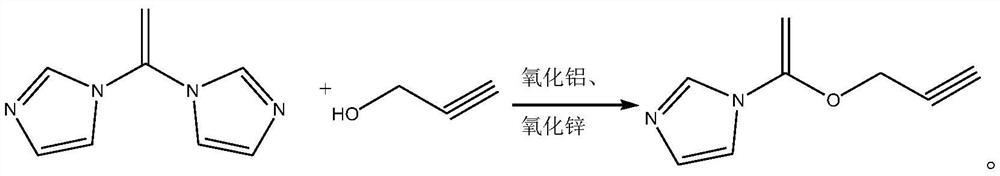
Synthesis method of 2-propyn-1-yl 1H-imidazole-1-carboxylic ester
A synthesis method and carboxylic acid ester technology, applied in the direction of organic chemistry and the like, can solve the problems of incomplete preparation and purification methods, unsuitable for industrial production, and limited industrial production, etc., and achieve a process with few reaction steps, simple operation, and easy-to-obtain raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthetic method of 2-propyn-1-yl 1H-imidazole-1-carboxylate, its synthetic steps are as follows:
[0020] Take 6.16g (0.11mol) of 2-propynyl alcohol and add it to 30mL of dichloromethane, and use it as the dichloromethane solution of 2-propynyl alcohol for later use;
[0021] Under nitrogen protection, dissolve 16.2g (0.1mol) of N,N'-carbonyldiimidazole in 30mL of dichloromethane, add a catalyst composed of 0.65g of alumina and 0.97g of zinc oxide, cool down to 3°C, and slowly drop Add the dichloromethane solution of 2-propynyl alcohol. After the dropwise addition, first maintain the reaction at 3°C for 1h, raise the temperature to 10°C for 1h, then raise the temperature to 20°C for 1h, and finally raise the temperature to reflux for 1.5h. After the reaction is completed , the resulting system was lowered to room temperature, removed the catalyst (aluminum oxide and zinc oxide) by filtration, then adjusted the pH value to 1, and separated the phases. The obtained...
Embodiment 2
[0024] The synthetic method of 2-propyn-1-yl 1H-imidazole-1-carboxylate, its synthetic steps are as follows:
[0025] Take 6g (0.107mol) of 2-propynyl alcohol and add it to 25mL of dichloromethane, and use it as the dichloromethane solution of 2-propynyl alcohol for later use;
[0026] Under nitrogen protection, dissolve 16.2g (0.1mol) of N,N'-carbonyldiimidazole in 30mL of dichloromethane, add a catalyst consisting of 0.405g of alumina and 0.405g of zinc oxide, cool down to 5°C, and slowly drop Add the dichloromethane solution of 2-propynyl alcohol. After the dropwise addition, first maintain the reaction at 5°C for 0.8h, then raise the temperature to 15°C for 0.5h, then raise the temperature to 25°C for 0.6h, and finally raise the temperature to reflux for 2h. After completion, the resulting system was lowered to room temperature, and the catalyst (aluminum oxide and zinc oxide) was removed by filtration, then the pH value was adjusted to 1.5, and the phases were separated. ...
Embodiment 3
[0028] The synthetic method of 2-propyn-1-yl 1H-imidazole-1-carboxylate, its synthetic steps are as follows:
[0029] Take 5.88g (0.105mol) of 2-propynyl alcohol and add it to 25mL of dichloromethane, and use it as the dichloromethane solution of 2-propynyl alcohol for later use;
[0030] Under nitrogen protection, dissolve 16.2g (0.1mol) of N,N'-carbonyldiimidazole in 30mL of dichloromethane, add a catalyst composed of 0.486g of alumina and 0.972g of zinc oxide, cool down to 0°C, and slowly drop Add the dichloromethane solution of 2-propynyl alcohol. After the dropwise addition, first maintain the reaction at 0°C for 0.9h, raise the temperature to 13°C for 0.8h, then raise the temperature to 23°C for 0.5h, and finally raise the temperature to reflux for 1.6h. After the reaction was completed, the resulting system was cooled to room temperature, and the catalyst (aluminum oxide and zinc oxide) was removed by filtration, then the pH value was adjusted to 1.3, and the phases wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com