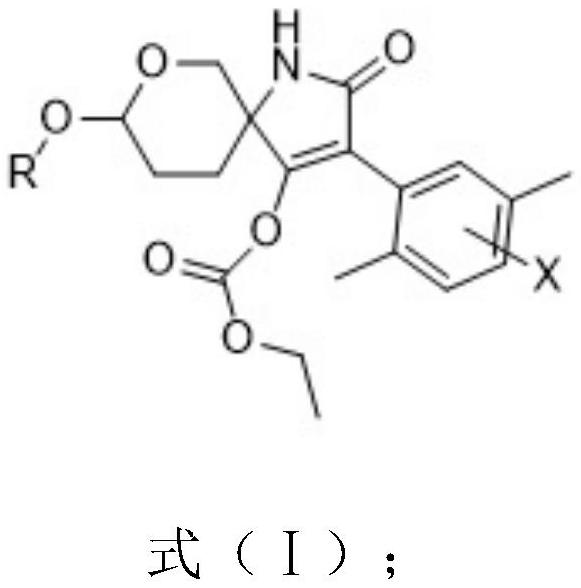
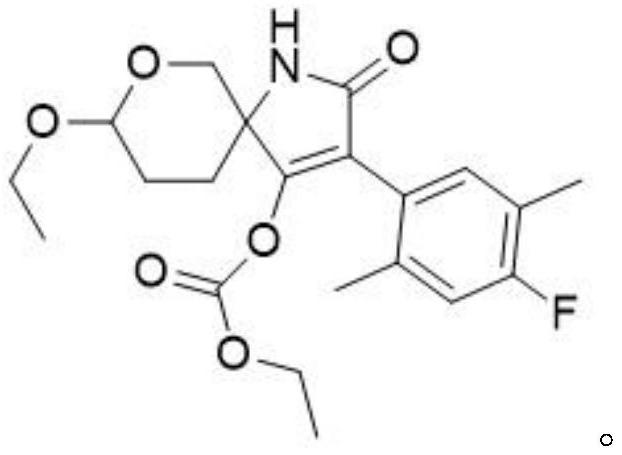
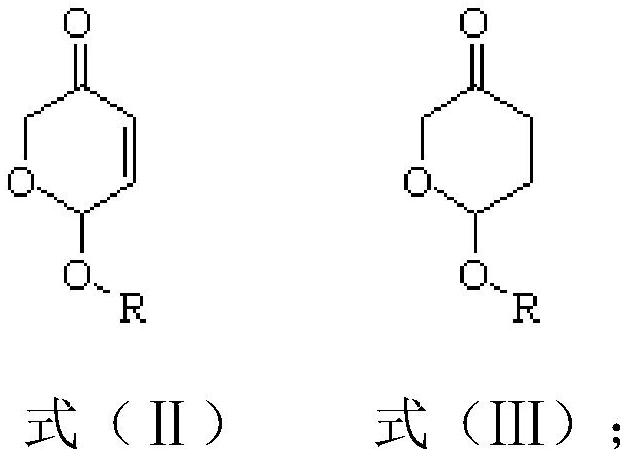
Spiroheterocyclic tetrahydropyran compound as well as preparation method and application thereof
A technology for pyran compounds and spiro heterocycles, which is applied in the field of spiro heterocycle tetrahydropyran compounds and their preparation, and can solve problems such as drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] This embodiment provides a preparation method of the compound shown in formula (III):
[0082] Add 500g of dichloromethane and 68.7g of furfuryl alcohol (0.7mol) into a 1L four-neck flask, control the temperature at 0-5°C, add 173g (1.0mol) of m-chloroperoxybenzoic acid, keep stirring for 6 hours, remove insoluble matter by filtration, Control the temperature of the obtained clarified mother liquor at 0-5°C, add 80.8g (0.8mol) of triethylamine, stir for 10min, slowly drop in 60.4g (0.77mol) of acetyl chloride, keep it warm for 2h after the dripping, and then pour into the reaction solution Add 50 mL of saturated sodium bicarbonate solution, stir for 30 min, separate the layers, extract the aqueous layer with 30 mL of dichloromethane x 2 times, combine the organic phases, and dry over anhydrous magnesium sulfate to obtain 5-carbonyl-5,6-dihydro-2H- A solution of pyran-2-yl acetate in dichloromethane;
[0083] Add 84 g (1.4 mol) of isopropanol to the dried 5-carbonyl-5,6...
Embodiment 2-3
[0091] Compound IIIb and Compound IIIc were synthesized by referring to the above-mentioned method, and the specific process parameters can be obtained by routine adjustment in accordance with Example 1.
[0092]
Embodiment 4
[0094] The preparation of compound shown in formula (IV):
[0095] Weigh 120 g (1.52 mol) of ammonium bicarbonate, add 20 g (0.4 mol) of sodium cyanide into a mixed solution of 30 g of water and 100 g of tetrahydrofuran, stir and suspend, add 60.6 g (0.38 mol) of compound IIa prepared in Example 3 at room temperature, After mixing evenly, raise the temperature to 55°C and keep it warm for 6h, filter, wash the filter cake with 20g of tetrahydrofuran, combine the mother liquors, concentrate to dryness, and dry to obtain 85.7g of white solid (Compound IVa), with a yield of 98% and a purity of 96.2%.
[0096]
[0097] Compound IVb and Compound IVc were synthesized by referring to the above-mentioned method, and the specific process parameters could be obtained by routine adjustment according to the above-mentioned examples.
[0098]
[0099]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com