Synthesis process of anti-heart-failure medicine sacubitril
A synthetic process, the technology of sacubitril, which is applied in the preparation of organic compounds, organic chemistry, carboxylic acid amide preparation, etc., can solve the problems of unfavorable scale-up production and high cost, and achieve low pollution, low cost, and cumbersome preparation The effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
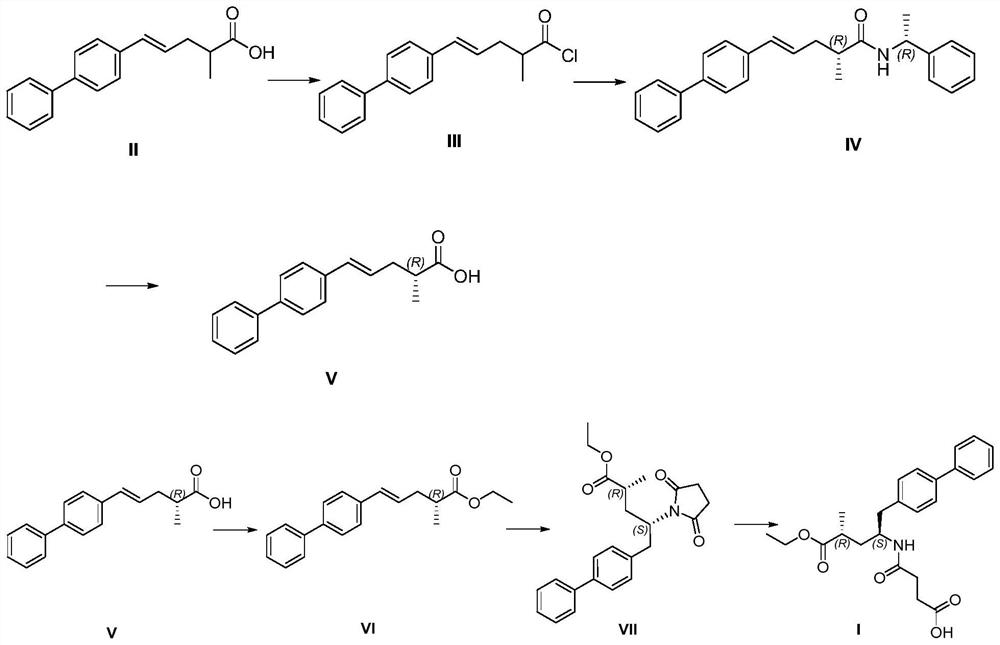
[0038] A synthesis process of anti-heart failure drug sacubitril, the specific steps of the synthesis process are:
[0039] (1) Preparation of compound V
[0040] S1, add 266g (1mol) of compound II, 1000ml of dichloromethane, 143g (1.2mol) of thionyl chloride to the reaction flask, heat up to 40°C under reflux and stir for 4 hours, follow the reaction by HPLC until the reaction of compound II is complete; concentrate In the reaction solution, 298 g of compound III was obtained, and the molar yield was 105%.
[0041] S2, add 1000ml of dichloromethane, 298g (1mol) of compound III, 121g (1.2mol) of triethylamine to the reaction flask, add 121g of R-phenylethylamine (1mol) dropwise at 20°C, stir for 2 hours after dropping, follow the reaction by HPLC Until the reaction of compound III is complete; the organic layer is washed with 1M dilute hydrochloric acid, washed with saturated sodium chloride, concentrated, and 147 g of compound IV is obtained by adding 1000 ml of toluene for ...
Embodiment 2
[0052] A synthesis process of anti-heart failure drug sacubitril, the specific steps of the synthesis process are:
[0053] (1) Preparation of compound V
[0054] S1, add 266g (1mol) compound II, 1000ml dichloromethane, 131g (1.1mol) sulfur oxychloride in the reaction flask, add temperature and reflux and stir for 6 hours, HPLC follows reaction until compound II reacts completely; Concentrate the reaction solution, 297.8 g of compound III was obtained, molar yield: 105%.
[0055] S2, add 1000ml dichloromethane, 297.8g (1mol) compound III, 101g (1.0mol) triethylamine to the reaction flask, add 121g R-phenylethylamine (1mol) dropwise at 30°C, stir for 6 hours after dropping, HPLC Track the reaction until the reaction of compound III is complete; wash the organic layer with 1M dilute hydrochloric acid, wash with saturated sodium chloride, concentrate, add 1000ml of toluene for beating to obtain 146.5g of compound IV, racemize the mother liquor with aqueous sodium hydroxide solut...
Embodiment 3
[0064] A synthesis process of anti-heart failure drug sacubitril, the specific steps of the synthesis process are:
[0065] (1) Preparation of compound V
[0066] S1, add 266g (1mol) compound II, 1000ml dichloromethane, 131g (1.1mol) sulfur oxychloride in the reaction bottle, add temperature and reflux and stir for 2 hours, HPLC follows reaction until compound II reacts completely; Concentrate the reaction solution, 298g of compound III was obtained, molar yield: 105%.
[0067] S2, add 1000ml dichloromethane, 298g (1mol) compound III, 111g (1.1mol) triethylamine to the reaction flask, add 121g R-phenylethylamine (1mol) dropwise at 40°C, stir for 4 hours after dropping, follow by HPLC React until the reaction of compound III is complete; wash the organic layer with 1M dilute hydrochloric acid, wash with saturated sodium chloride, concentrate, add 1000ml of toluene for beating to obtain 147g of compound IV, add aqueous sodium hydroxide solution to racemize the mother liquor, ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com