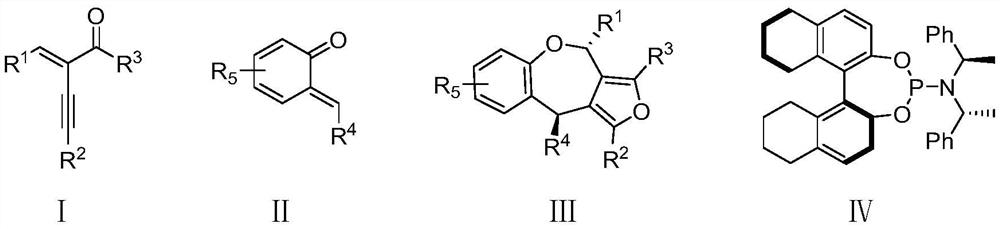
Method for synthesizing chiral tetrahydrobenzoxepine compound through asymmetric cycloaddition reaction catalyzed by gold
A technology of benzoxazepines and compounds, applied in the field of organic synthesis, to achieve the effects of convenient and simple operation, mild reaction conditions, and a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of chiral ligand used in the embodiment is as follows:
[0047] At 0-5°C, slowly add 5.8 mL of triethylamine dropwise to 50 mL of 0.2 mol / L phosphorus trichloride in dichloromethane for 5 minutes, and then add VI (10 mmol, 2.25 g) 20 mL of dichloromethane solution was added dropwise to the reaction solution. After the dropwise addition was completed, it was warmed up to room temperature and continued to stir at room temperature for 2 h. Then, compound VII (10 mmol, 2.94 g) was added and stirred at room temperature overnight. After the reaction was completed, the solvent was removed in vacuo. EA / PE=0.02-0.04:1, v / v), the target product IV was obtained (71% yield, 99% ee).
[0048] The reaction scheme is as follows:
[0049]
Embodiment 1
[0051] (6R,10S)-7-Methyl-6,9-diphenyl-10-((E)-styryl)-6H,10H-[1,3]dioxacyclo[4',5' : Synthesis of 4,5]benzo[1,2-b]furo[3,4-E]oxazepine(IIIaa)
[0052] The reaction scheme is as follows:
[0053]
[0054] The operation steps are as follows:
[0055] Under nitrogen protection, chiral ligand (7.5 μmol, 4.1 mg), Me 2 SAuCl (5 μmol, 1.5 mg) and 0.5 mL of dichloroethane were stirred at room temperature for 2 hours, and the solvent was removed in vacuo. Next the AgNTf 2 (5.5μmol, 2.2mg) and 1.0mL dichloroethane were added to the reaction flask respectively, after the mixture was stirred at room temperature for 15 minutes, the precipitate was removed by filtration, and the filtrate was transferred to a container containing substrate Ia (0.1mmol, 24.6mg), substrate Ⅱa (0.13mmol, 32.8mg) and 300mg Molecular sieve reaction flask. Then stirred at room temperature for 3 hours, TLC detection, the raw material Ia disappeared; after that, the solvent was removed by concentration und...
Embodiment 2
[0062] (6R,10S)-7-(4-fluorophenyl)-6,9-diphenyl-10-((E)-styryl)-6H,10H-[1,3]dioxane[ Synthesis of 4',5':4,5]benzo[1,2-b]furo[3,4-E]oxazepine(Ⅲba)
[0063] The reaction scheme is as follows:
[0064]
[0065] The operation steps are as follows:
[0066] Under nitrogen protection, chiral ligand (7.5 μmol, 4.1 mg), Me 2 SAuCl (5 μmol, 1.5 mg) and 0.5 mL of dichloroethane were stirred at room temperature for 2 hours, and the solvent was removed in vacuo. Next the AgNTf 2 (5.5μmol, 2.2mg) and 1.0mL dichloroethane were added to the reaction flask respectively, and after the mixture was stirred at room temperature for 15 minutes, the precipitate was removed by filtration, and the filtrate was transferred to a solution containing substrate Ib (0.1mmol, 26.4mg), substrate IIa (0.13mmol, 32.8mg) and 300mg Molecular sieve reaction flask. Then stirred at room temperature for 3 hours, TLC detection, the raw material Ib disappeared; after that, the solvent was removed by concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com