Bionic system and method for in-vitro regulation and control of inorganic salt crystallization
A bionic system and inorganic salt technology, applied in chemical instruments and methods, separation methods, crystallization separation, etc., to achieve the effect of wide application range, easy operation, and easy construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
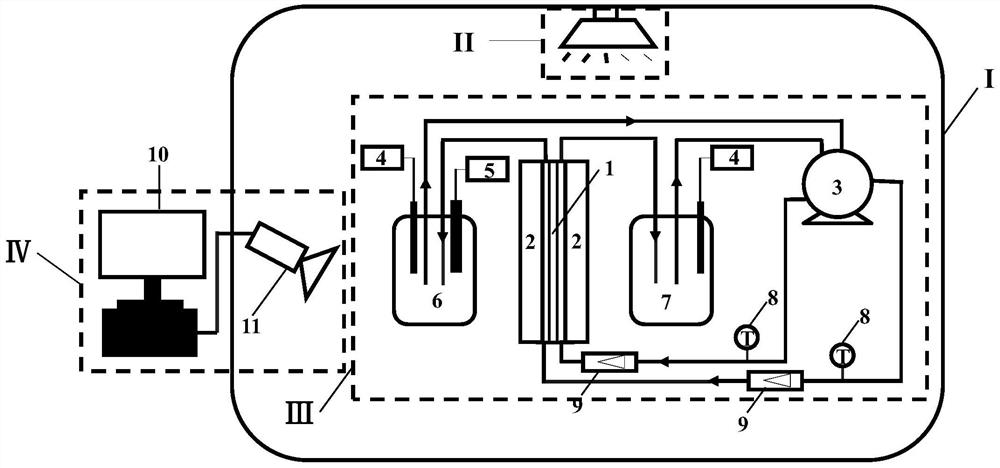
[0038] (1) Design and manufacture a membrane module 2 similar in structure to the human knee joint;
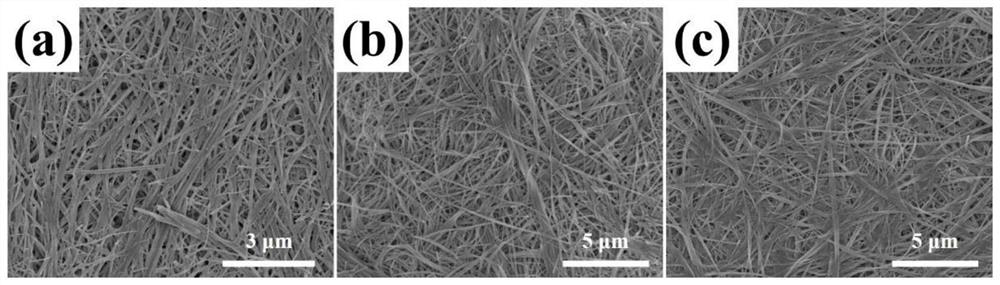
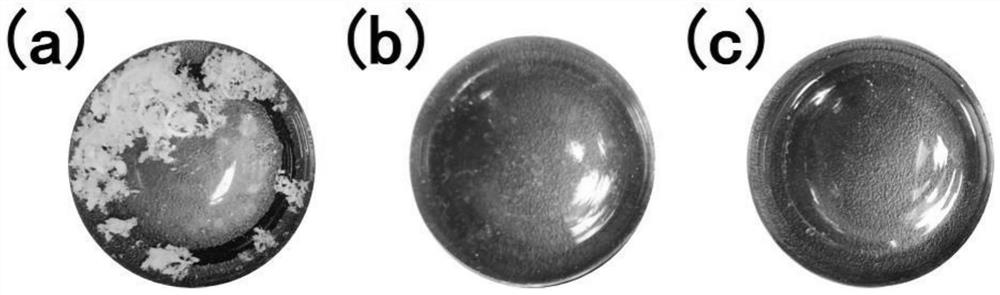
[0039] (2) Prepare non-toxic EGDMA-HEMA flat membrane 1 with good biocompatibility and mechanical properties similar to hyaline cartilage, the molar ratio of which is 3:5, and cut it into the size and shape matched with the membrane module 2;
[0040] (3) Assembling the membrane module 2 and the EGDMA-HEMA flat membrane 1 together;
[0041] (4) Prepare 2 bottles of 100mL simulated body fluid with a sodium ion concentration of 140mM and a pH of 7.4, and add 1.5mM uric acid to one of the bottles as a crystallization solution;
[0042] (5) Set the temperature of the environment where the inorganic salt crystallization module III is located, use the constant temperature module I to maintain the temperature at 20°C, and monitor it in real time through the thermometer 8;
[0043] (6) Pass the liquid outlet pipes of the crystallization liquid tank 6 and the simulated body fluid tank...
Embodiment 2
[0048] (1) Design and manufacture a membrane module 2 similar in structure to the human knee joint;
[0049] (2) Prepare non-toxic, good biocompatibility and hyaline cartilage mechanical properties similar to the EGDMA-HEMA-AA flat membrane 1, the molar ratio is 2:5:3, and cut it to match the membrane module 2 size and shape;
[0050] (3) Assembling the membrane module 2 and the EGDMA-HEMA-AA flat membrane 1;
[0051] (4) Prepare 2 bottles of 100mL simulated body fluid with a sodium ion concentration of 140mM and a pH of 7.4, and add 1.5mM uric acid to one of the bottles as a crystallization solution;
[0052] (5) Set the temperature of the environment where the inorganic salt crystallization module III is located, use the constant temperature module I to maintain the temperature at 20°C, and monitor it in real time through the thermometer 8;
[0053] (6) Through the polyvinyl chloride transparent hose, the liquid outlet pipes of the crystallization liquid tank 6 and the sim...
Embodiment 3
[0058] (1) Design and manufacture a membrane module 2 similar in structure to the human knee joint;
[0059] (2) Prepare non-toxic, good biocompatibility and hyaline cartilage mechanical properties similar to the EGDMA-AA flat film 1, the molar ratio of which is 3:5, and cut it into the size and shape matched with the film component 2;
[0060] (3) Assembling the membrane module 2 and the EGDMA-AA flat membrane 1;
[0061] (4) Prepare 2 bottles of 100mL simulated body fluid with a sodium ion concentration of 140mM and a pH of 7.4, and add 1.5mM uric acid to one of the bottles as a crystallization solution;
[0062] (5) Set the temperature of the environment where the inorganic salt crystallization module III is located, use the constant temperature module I to maintain the temperature at 20°C, and monitor it in real time through the thermometer 8;
[0063] (6) Through the polyvinyl chloride transparent hose, the liquid outlet pipes of the crystallization liquid tank 6 and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com