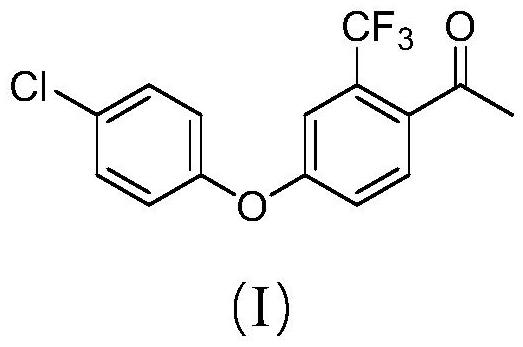
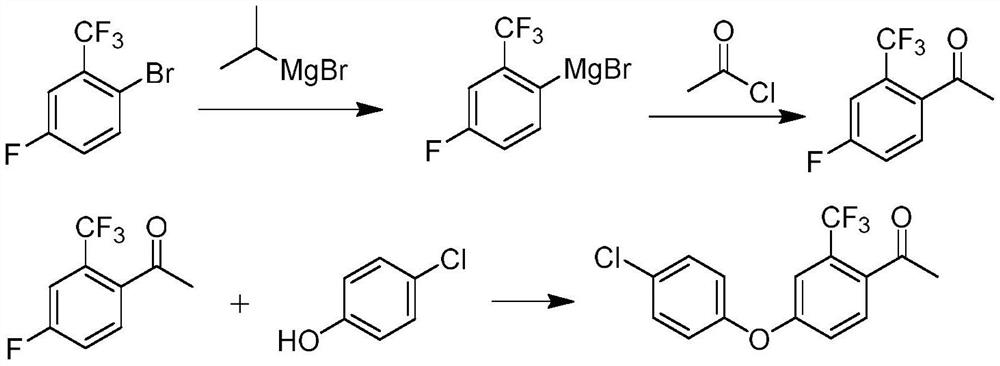
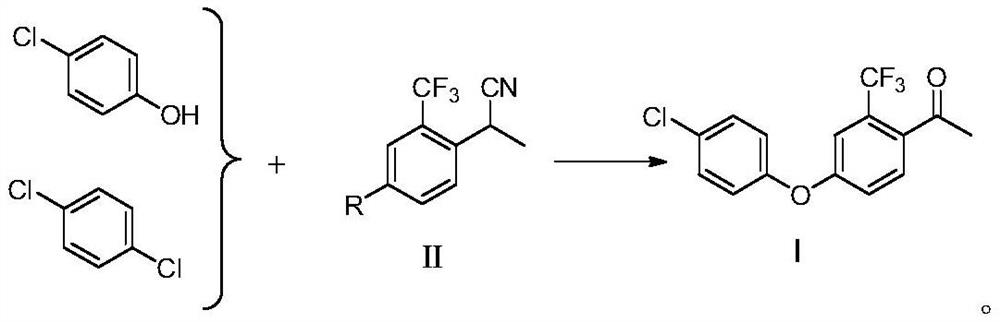
Preparation method of meconazole intermediate
A technology of chloroflufeconazole and intermediates, which is applied in the field of preparation of intermediate compounds, can solve the problems of large amount of three wastes, high cost, and harsh production environment, so as to improve yield and purity, reduce production costs, and avoid reaction conditions harsh effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 4-(4-chlorophenoxy)-2-trifluoromethylacetophenone
[0045] In a four-necked flask equipped with a mechanical stirring, a thermometer and a condenser, add 25.7g (0.1mol, 95%) of formula (II) and 150mL DMF, then add 15.4g (0.12mol, 99%) p-chlorophenol, 16.8g (0.12mol, 99%) potassium carbonate and 0.4g copper acetate, heat up at 120-125°C, blow in air and stir for 5h;
[0046] HPLC detects that the raw materials are complete, the recovered solvent is added with toluene, acid water is added to adjust pH=7, the aqueous phase is separated, and the organic layer is removed from toluene to obtain 4-(4-chlorophenoxy)-2-trifluoromethylacetophenone 29.8 g, content 98%, yield 95%.
Embodiment 2
[0048] The difference from Example 1 is that in the step, the nitro group of formula (II) is replaced by a fluorine group, other conditions remain unchanged, the content is 98%, and the yield is 89%.
Embodiment 3
[0050] The difference from Example 1 is that in the step, the nitro group of formula (II) is replaced with a hydroxyl group, and p-chlorophenol is replaced with p-dichlorobenzene, other conditions remain unchanged, the content is 97%, and the yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com