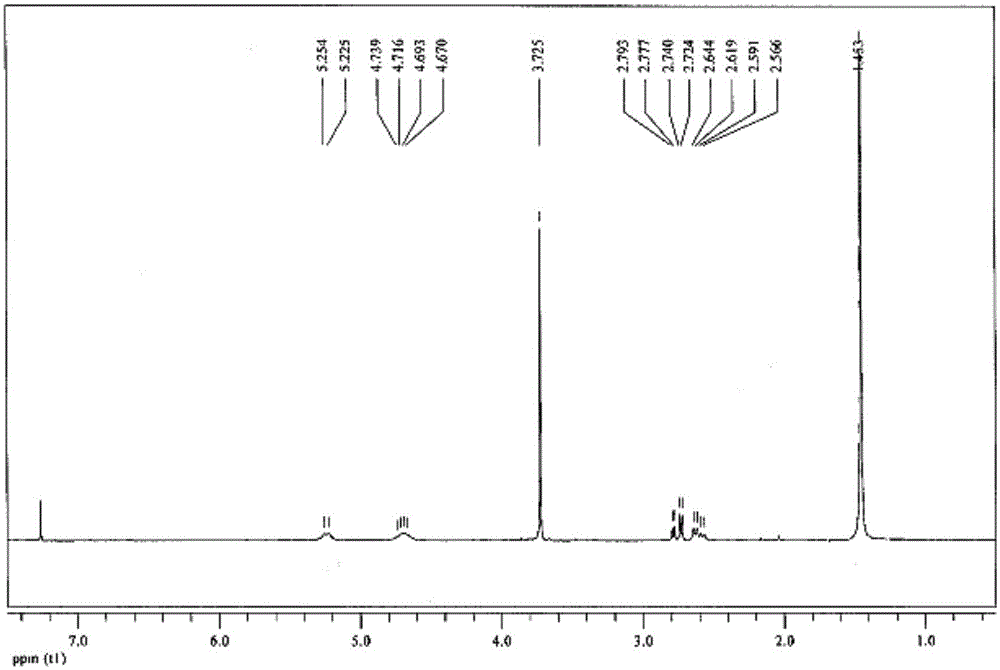
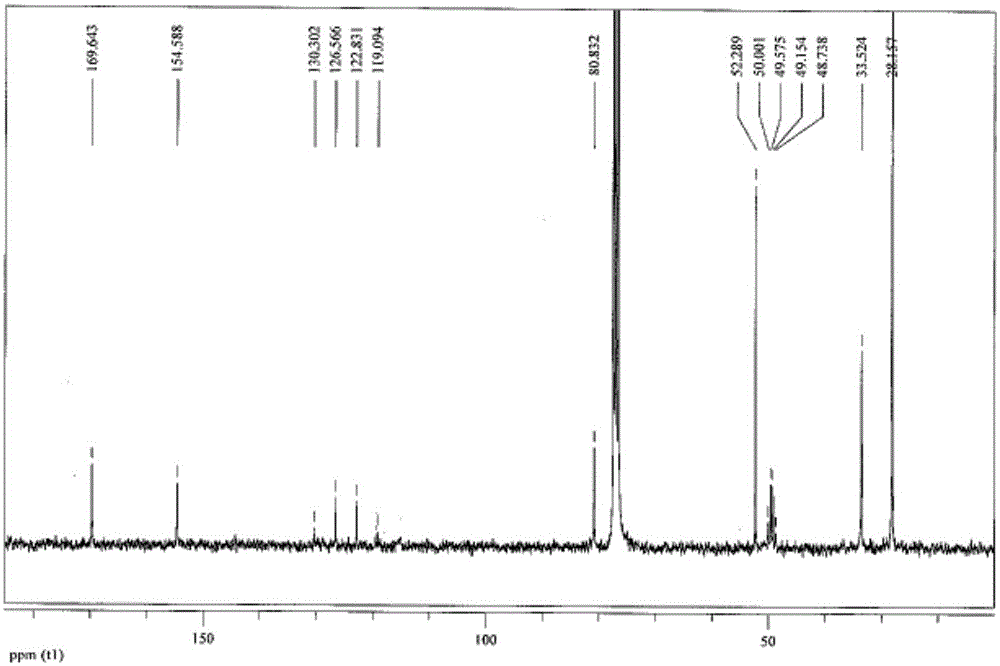
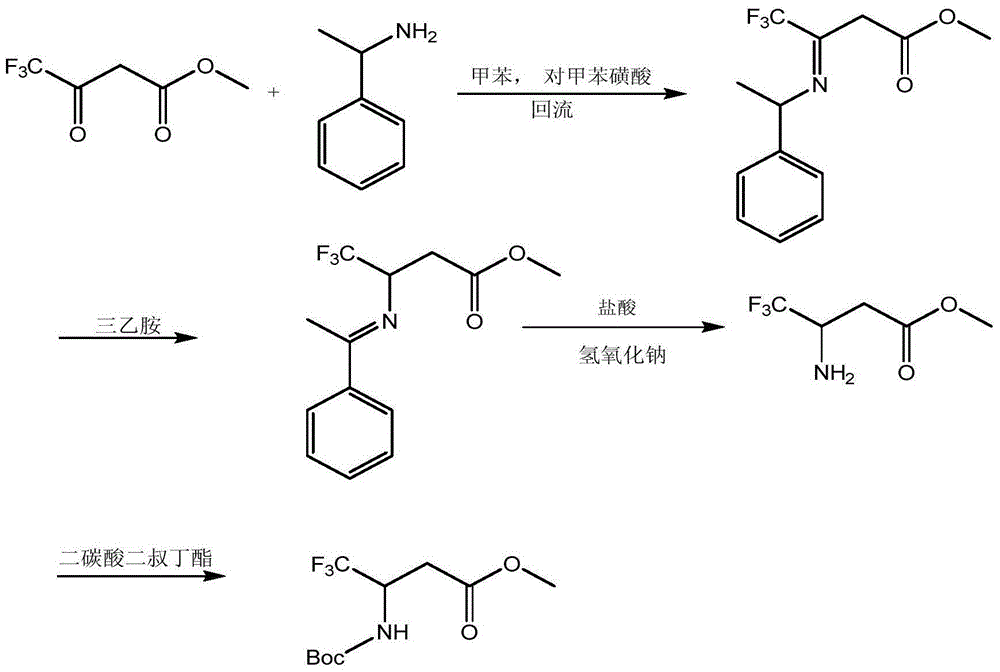
A kind of preparation method of 3-tert-butoxyamido-4,4,4-trifluorobutyric acid methyl ester
A technology of tert-butoxyamido and methyl trifluorobutyrate, which is applied in the field of preparation of 3-tert-butoxyamido-4,4,4-methyl trifluorobutyrate, can solve the problem of harsh reaction conditions and raw materials. The problems of high price and many by-products can achieve the effect of low reaction temperature, easy availability of raw materials and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] At -20°C, methyl 4,4,4-trifluoro-3-oxobutyrate (1.701g, 0.01mol) was added to the organic solvent ether (8.5ml), and then triethylamine (1.012g ,0.01mol), acetic anhydride (1.021g, 0.01mol), and then add tert-butyl carbamate (1.172g, 0.01mol); the reaction was slowly raised to 20°C (3-5°C / min), and kept for 1h; Filtrate, concentrate the filtrate under normal pressure to obtain a white solid; dry in a vacuum oven at 40°C for 6 hours, add the dried product from the previous step to an autoclave, add 8.5ml of methanol solvent to dissolve, then add 0.0085g of palladium carbon catalyst, hydrogenation For reduction, the temperature is 30° C., and the reaction time is 4 hours. After the reduction is completed, filter, and the filtrate is distilled off the solvent under normal pressure to obtain the product. The yield is 80%, and the purity detected by gas chromatography is 99%. Product melting point: 60~62℃.
Embodiment 2
[0026] At 0°C, methyl 4,4,4-trifluoro-3-oxobutyrate (1.701g, 0.01mol) was added to the organic solvent ether (17ml), and then triethylamine (5.060g, 0.05 mol), acetic anhydride (5.105g, 0.05mol), and then add tert-butyl carbamate (8.505g, 0.05mol); slowly raise the reaction to 50°C (3-5°C / min), keep the reaction for 4h; filter, Concentrate the filtrate under normal pressure to obtain a white solid; dry it in a vacuum oven at 40°C for 6 hours, add the dried product from the previous step to an autoclave and add 17ml of methanol solvent to dissolve, then add 0.085g of palladium carbon catalyst, hydrogenation reduction, temperature The temperature is 60°C, the reaction time is 8h, after the reduction is completed, filter, and the filtrate is distilled off the solvent under normal pressure to obtain the product. The yield is 95%, and the purity detected by gas chromatography is 99.3%.
Embodiment 3
[0028] At -10°C, methyl 4,4,4-trifluoro-3-oxobutyrate (1.701g, 0.01mol) was added to the organic solvent tetrahydrofuran (10.2ml), and then pyridine (1.582g, 0.02 mol), acetic anhydride (2.042g, 0.02mol), then add tert-butyl carbamate (2.344g, 0.02mol); slowly raise the reaction to 30°C (3~5°C / min), keep the reaction for 2h; filter, Concentrate the filtrate under normal pressure to obtain a white solid; dry it in a vacuum oven at 40°C for 6 hours, add the dried product from the previous step to an autoclave and add 10.2ml of ethanol solvent to dissolve it, then add 0.017g of palladium carbon catalyst, hydrogenation and reduction, The temperature is 40°C, the reaction time is 5h, after the reduction is completed, filter, and the filtrate is distilled off the solvent under normal pressure to obtain the product. The yield was 83.3%, and the purity detected by gas chromatography was 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com