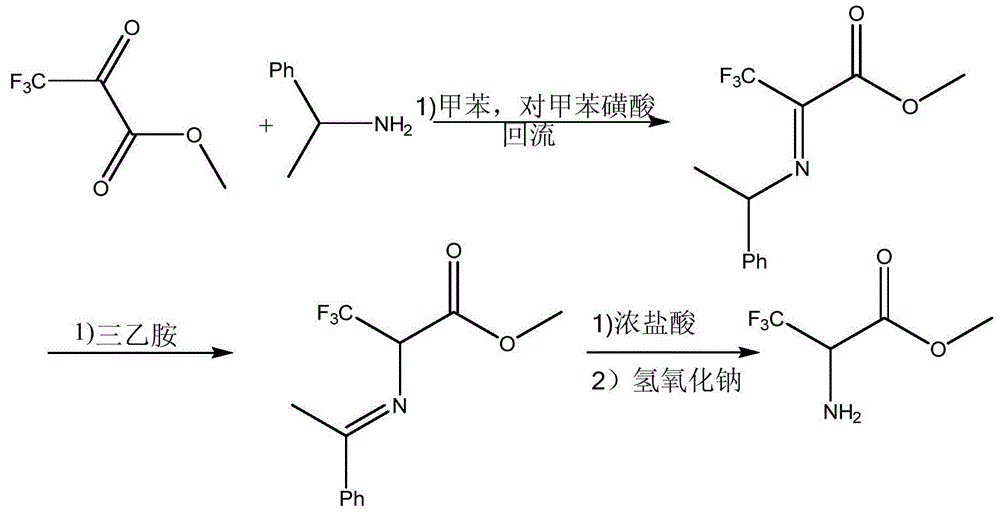
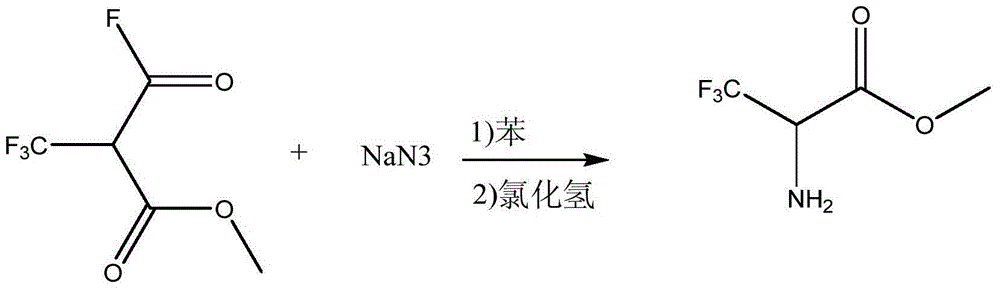
Preparation method of methyl trifluoroalaninate
A technology of methyl trifluoroalanine and methyl trifluoropyruvate, which is applied in the field of organic synthesis, can solve the problems of sodium azide being inflammable and explosive, no economical and effective report, harsh reaction conditions, etc., and achieves easy operation. , the effect of short time and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
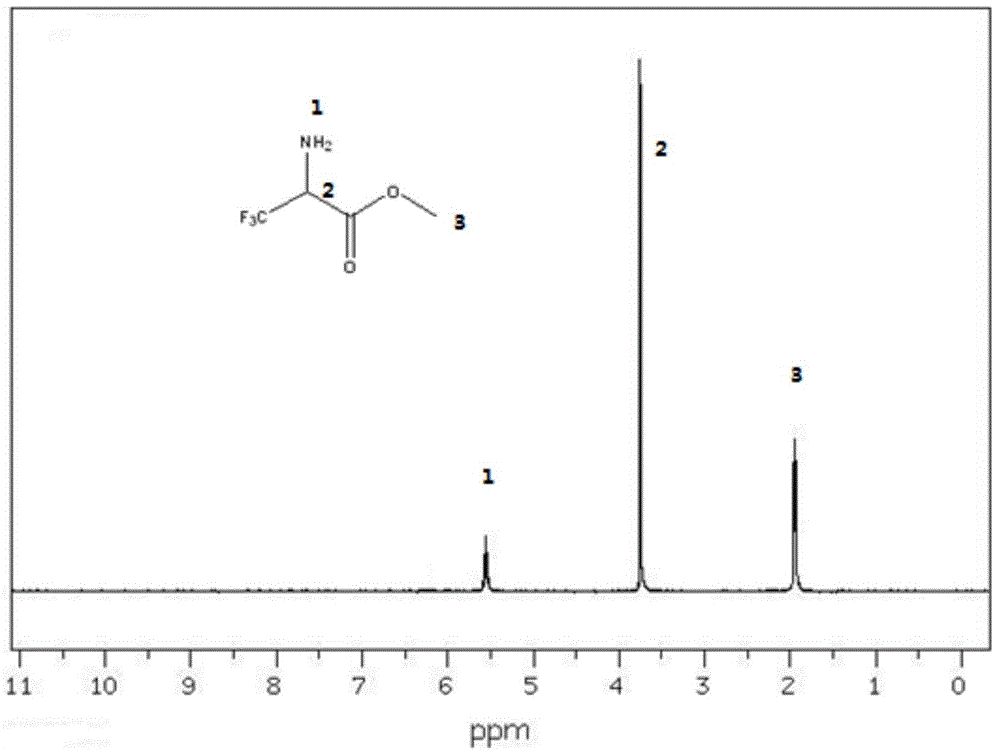
[0022] At 0°C, methyl trifluoropyruvate (15.6 g, 0.1 mol) and 15.6 mL of toluene were added to a four-necked flask, sodium acetate (8.2 g, 0.1 mol), and hydroxylamine hydrochloride (6.9 g, 0.1 mol) were added. The reaction was slowly heated to reflux (about 110 degrees), and the reaction was kept for 1 hour. The temperature of the reaction solution was lowered to 30 degrees, p-toluenesulfonic acid (1.7g, 0.01mol) was added, heated to reflux (110°C), and reacted for 1 hour. After the toluene was distilled off under reduced pressure, the reaction liquid was poured into a mixture of ice and water, and a large amount of solid was precipitated, which was filtered. After the filtered solid was dried at 50°C for 10 hours, it was added to the autoclave, 0.078g of palladium carbon catalyst was added, 78ml of ethanol was added to dissolve, hydrogenation reduction, 20°C, react for 3 hours, filter after the completion of the reaction, the filtrate is always The solvent is distilled off un...
Embodiment 2
[0025] At 15℃, add methyl trifluoropyruvate (15.6g, 0.1mol) and 78.0mL xylene into a four-necked flask, add sodium hydroxide (12.0g, 0.3mol), hydroxylamine hydrochloride (20.7g, 0.3mol) . The reaction was slowly heated to reflux (about 130 degrees), and the reaction was kept for 2 hours. The reaction solution was cooled to 30 degrees, and p-toluenesulfonic acid (5.1g, 0.03mol) was added, heated to reflux (130°C), and reacted for 2h. After the xylene was distilled off under reduced pressure, the reaction liquid was poured into a mixture of ice and water, and a large amount of solid was precipitated, which was filtered. After the filtered solid was dried at 50°C for 10 hours, added to the autoclave, added 0.078 g of catalyst palladium carbon, added 78 ml of methanol to dissolve, hydrogenated reduction, 50°C, reacted for 4 hours, filtered after the reaction is complete, the filtrate is usually The solvent is distilled off by pressure, the obtained crude product is distilled unde...
Embodiment 3
[0027] At 30℃, add methyl trifluoropyruvate (15.6g, 0.1mol) and 124.8mL methyl acetate into a four-necked flask, add sodium bicarbonate (42.0g, 0.5mol), hydroxylamine hydrochloride (34.5g, 0.5mol) ). The reaction was slowly heated to reflux (approximately 57 degrees), and the reaction was kept for 4 hours. The reaction solution was cooled to 30 degrees, and p-toluenesulfonic acid (8.5g, 0.05mol) was added, heated to reflux (57°C), and reacted for 4h. After the methyl acetate was distilled off under reduced pressure, the reaction liquid was poured into a mixture of ice and water, and a large amount of solid was precipitated, which was filtered. After the filtered solid was dried at 50°C for 10 hours, it was added to the autoclave, 0.78 g of palladium carbon catalyst was added, and 156 ml of ethyl acetate was added to dissolve it. Hydrogenation reduction was carried out at 80°C for 5 hours. After the reaction was completed, it was filtered. The solvent is evaporated from the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com