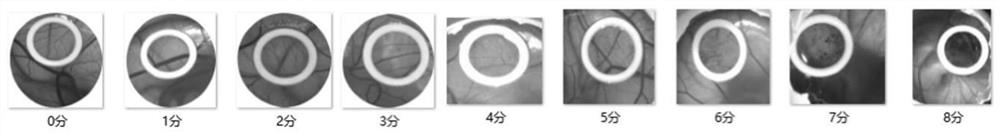
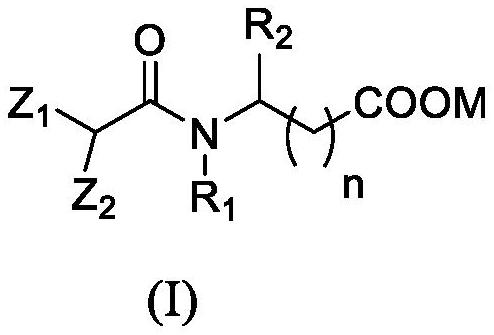
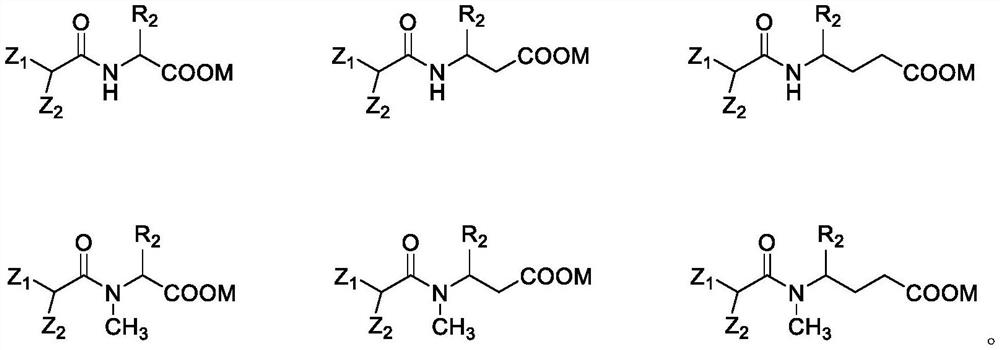
Isopalmitoyl amino acid compound as well as preparation method and application thereof
A palmitoyl amino acid and amino acid technology, which is applied in the preparation of organic compounds, the preparation of carboxylic acid amides, chemical instruments and methods, etc., can solve the problems of poor compatibility, high irritation, and refractory degradation, and achieve high water solubility and irritation. The effect of low sex and strong water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0122] In a second aspect of the present invention, there is provided a preparation method of an isopalmitoyl amino acid compound, comprising the following steps:
[0123] S100: Using isopalmitic acid as a raw material, adding a chlorinating reagent to carry out an acid chlorination reaction to prepare isopalmitoyl chloride.
[0124] S200: Mix the amino acid compound, acid binding agent and solvent, add the isopalmitoyl chloride to carry out amidation reaction to prepare isopalmitoyl amino acid compound; wherein, the structure of the amino acid compound is NH 2 -CH(R 2 )-COOH, where R 2 As defined in the first aspect of the present invention.
[0125] S100: Preparation of isopalmitoyl chloride
[0126] Isopalmitoyl chloride can be prepared by the following preparation steps: adding isopalmitic acid and n-heptane to mix, and adding a chlorination reagent dropwise to the reaction flask under stirring conditions; Chromatography TLC could not be detected); the reaction solutio...
Embodiment 1
[0153] 1.1 Preparation of isopalmitoyl chloride
[0154] Add isopalmitic acid (0.39mol, 100g) and n-heptane (150mL) to a 500mL reaction flask and mix, under stirring, cool down to 0°C to 10°C, dropwise add oxalyl chloride (64.4g, 0.507°C) to the reaction flask mol), during the dropping process, maintain the reaction temperature at 0 ℃~10 ℃; after about 3-4 hours of dripping, be warming up to 20 ℃~30 ℃, continue to react for 1-2 hours isopalmitic acid conversion is completed; Concentrate under reduced pressure below 55°C until no solvent is evaporated to obtain isopalmitoyl chloride (0.383mol, 105.2g) as a pale yellow liquid with a yield of 98% and a purity of 97%.
[0155] 1.2 Preparation of isopalmitoyl aspartate
[0156] Add aspartic acid (0.109mol, 14.5g) and water (80mL) to a 250mL reaction flask and mix, add sodium hydroxide (0.109mol, 4.4g) under stirring to prepare an aqueous solution of sodium aspartate; add acetone (40mL) mixed, and dropwise added isopalmitoyl chlor...
Embodiment 2
[0160] 2.1 Preparation of isopalmitoyl chloride
[0161] Isopalmitoyl chloride was prepared by exactly the same preparation method as step 1.1 in Example 1.
[0162] 2.2 Preparation of isopalmitoyl sarcosine
[0163] To a 250mL reaction flask, add sarcosine (0.102mol, 9.1g) and water (80mL) to mix, add sodium hydroxide (0.102mol, 4.1g) under stirring to prepare sodium sarcosinate aqueous solution; add acetone (40mL) ) and mix, add isopalmitoyl chloride (0.073mol, 20g) and 32% aqueous sodium hydroxide solution (0.146mol, 18.3g) dropwise to the reaction flask. During the dropwise addition, maintain the pH of the reaction solution at 10 to 11 and the reaction temperature at 10 ℃~20℃, the dropwise addition is completed in about 2-3 hours; after the dropwise addition is completed, the reaction is continued for 1-2 hours until the raw material isopalmitoyl chloride is converted; hydrochloric acid is added to adjust the pH to 3~4, the acetone is concentrated and recovered, and left ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com