Fluorescent small molecule as well as preparation method and application thereof
A small molecule, fluorescent technology, applied in the fields of applications, chemical instruments and methods, luminescent materials, etc., can solve the problems of reducing the concentration of F127 and the adverse effects of loaded cell growth and migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
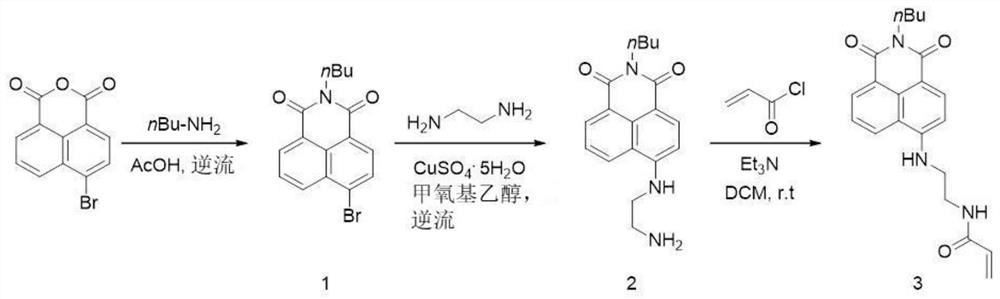
[0080] Step 1: Synthesis of Small Molecule A:
[0081] Step 1.1, synthesis of compound 1: 1.0 g of 4-bromo-1,8-naphthalene anhydride and 0.35 g of ammonia water were added to 8 ml of absolute ethanol, heated to boiling and stirred overnight. After the reaction was completed, it was cooled to room temperature, and the reaction solution was poured into 40 ml of ice water and filtered to obtain a brown solid. The dried solid was recrystallized with an appropriate amount of acetic acid to obtain pure compound 1 with a yield of 88%.
[0082] Step 1.2, the synthesis of compound 2: 0.5 g of compound 1 and 3.2 mL of ethylenediamine were dissolved in 7.6 mL of methoxymethanol, then 50 mg of copper sulfate pentahydrate was added, and the mixture was heated to boiling and stirred overnight. After cooling to room temperature, the reaction solution was poured into 100 ml of water and filtered to obtain a yellow solid. The dried solid was recrystallized from ethanol to obtain pure compound ...
Embodiment 2
[0095] Step 1: Synthesis of Small Molecule A:
[0096] Step 1.1, synthesis of compound 1: 4-Bromo-1,8-naphthalene anhydride (1.35 g, 5 mmol) and ammonia water (2.5 ml) were added to 150 ml of ethanol solution, heated to boiling and stirred overnight. After the reaction was completed, it was cooled to room temperature, and the reaction solution was filtered and rinsed with ethanol to obtain a brown solid. The dried solid was recrystallized with an appropriate amount of acetic acid to obtain pure compound 1 with a yield of 87%.
[0097]Step 1.2, the synthesis of compound 2: 0.5 g of compound 1 and 3.2 mL of ethylenediamine were dissolved in 7.6 mL of methoxymethanol, then 50 mg of copper sulfate pentahydrate was added, and the mixture was heated to boiling and stirred overnight. After cooling to room temperature, the reaction solution was poured into 100 ml of water and filtered to obtain a yellow solid. The dried solid was recrystallized from ethanol to obtain pure compound 2 w...
Embodiment 3
[0111] Step 1: Synthesis of Small Molecule B:
[0112] Step 1.1, synthesis of compound 1: 1.0 g of 4-bromo-1,8-naphthalene anhydride and 0.35 g of n-butylamine were added to 8 ml of acetic acid solution, heated to boiling and stirred overnight. After the reaction was completed, it was cooled to room temperature, and the reaction solution was poured into 40 ml of ice water and filtered to obtain a brown solid. The dried solid was recrystallized with an appropriate amount of acetic acid to obtain pure compound 1 with a yield of 85%.
[0113] Step 1.2, the synthesis of compound 2: 0.5 g of compound 1 and 3.2 mL of ethylenediamine were dissolved in 7.6 mL of methoxymethanol, then 50 mg of copper sulfate pentahydrate was added, and the mixture was heated to boiling and stirred overnight. After cooling to room temperature, the reaction solution was poured into 100 ml of water and filtered to obtain a yellow solid. The dried solid was recrystallized from ethanol to obtain pure compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com