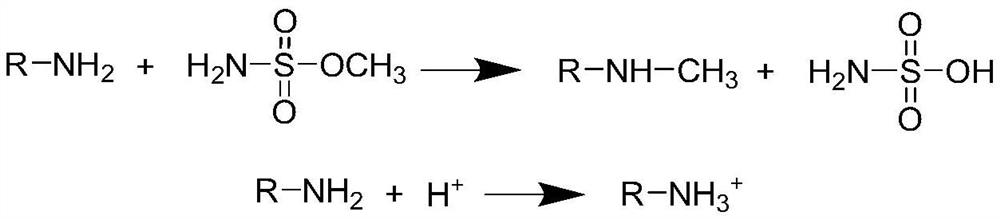
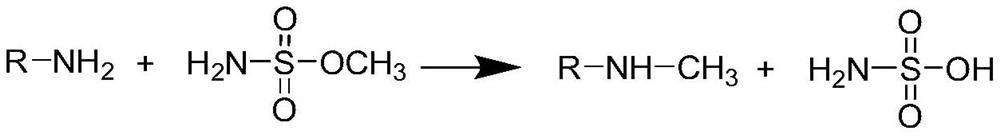
Method for detecting methyl sulfamate in free amino drug
A technology of methyl sulfamate and free amino groups, which is applied in the direction of measuring devices, material separation, instruments, etc., can solve the problems of difficult detection of methyl sulfamate, and achieve the advantages of quality control, good specificity, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1 Sample processing
[0061] 1.1 Reference substance stock solution: Take about 3 mg of methyl sulfamate reference substance, accurately weigh it, put it in a 10 mL volumetric flask, add methanol to dissolve and dilute to the mark, and shake well.
[0062] 1.2 Reference substance solution: Precisely measure 0.13mL of the reference substance stock solution, put it in a 10mL volumetric flask, add 10% formic acid solution to dilute to the mark, shake well, accurately measure 2mL, put it in a separatory funnel, and accurately add 4mL of ethyl acetate, After sufficient shaking and standing for separation, the ethyl acetate layer was taken.
[0063] 1.3 Test solution: Accurately weigh 800 mg of the sample, put it in a separatory funnel, add 2 mL of 10% formic acid solution, mix well, accurately add 4 mL of ethyl acetate, shake fully, and after standing for stratification, take the ethyl acetate layer. .
[0064] 2 Experimental process
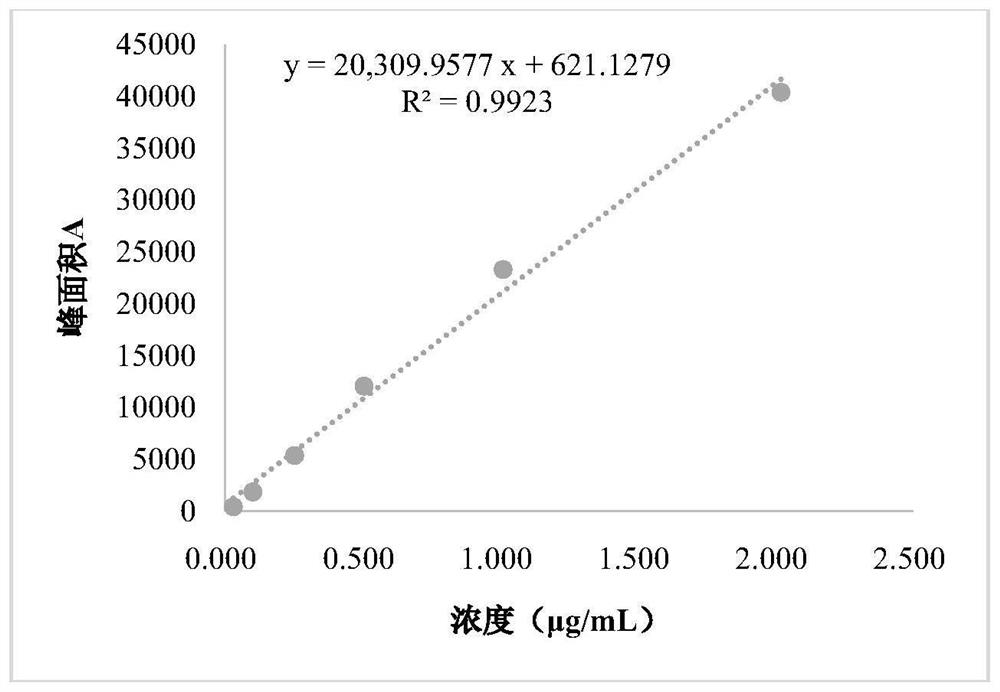
[0065] 2.1 Linear
[0066] Precisel...
Embodiment 2
[0089] The difference from Example 1 is that Example 2 is not treated with formic acid, and the solvent is methanol, and the operating process of detection is as follows:
[0090] 1 Sample processing
[0091] Reference substance stock solution: take about 3 mg of methyl sulfamate reference substance, accurately weigh it, place it in a 10 mL volumetric flask, add methanol to dissolve and dilute to the mark, shake well, accurately measure 0.3 mL, and place it in a 10 mL volumetric flask , dilute to volume with methanol and shake well.
[0092] Reference substance solution: Precisely measure 1mL of the reference substance stock solution, put it in a 10mL volumetric flask, dilute to the mark with methanol, and shake well.
[0093] Test solution: take about 200 mg of the test, accurately weigh it, put it in a centrifuge tube, add 2 mL of methanol accurately, ultrasonicate for 20 min, and centrifuge at 5000 r / min for 10 min to obtain the supernatant.
[0094] 100% recovery solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com