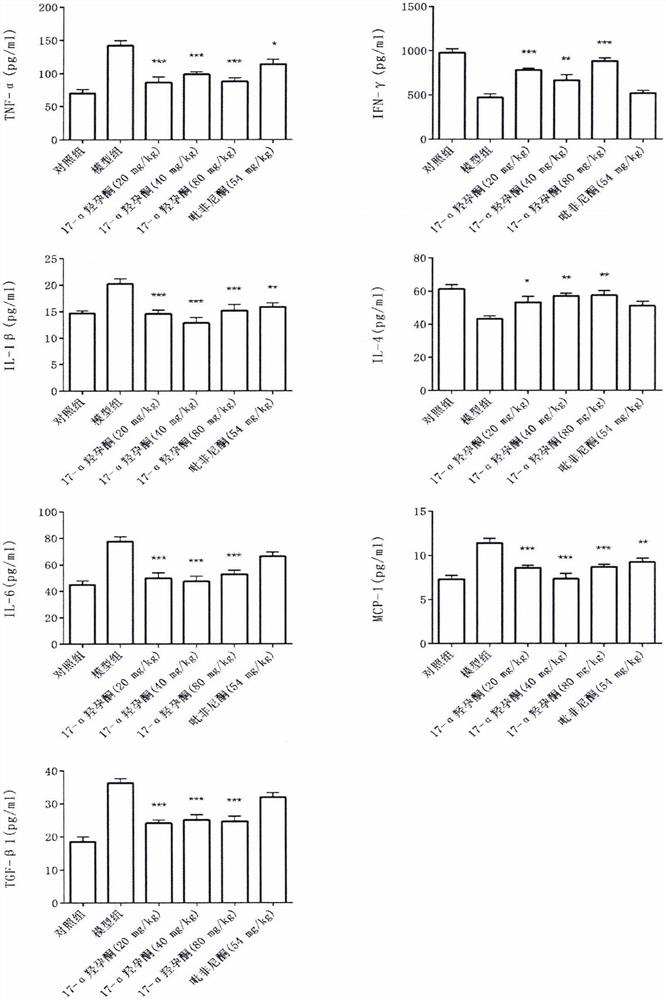
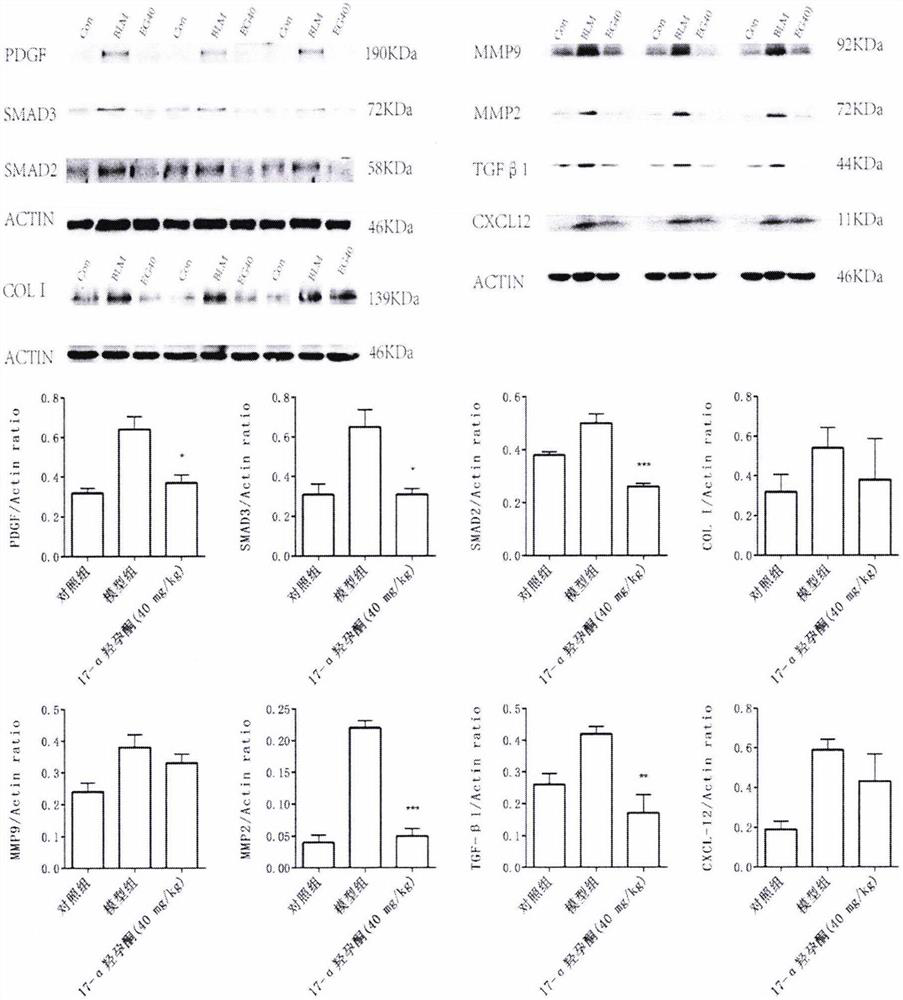
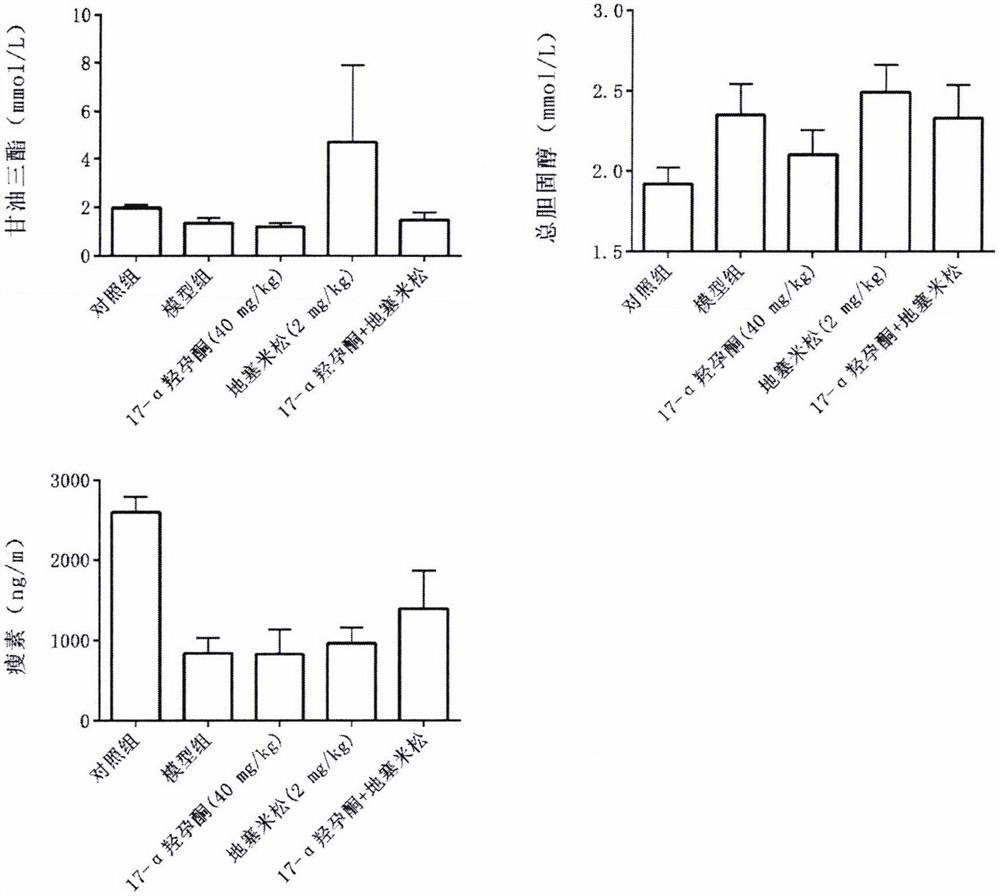
Application of hydroxyprogesterone hexanoate in preparation of medicine for treating interstitial pneumonia
A hydroxyprogesterone caproate, interstitial technology is applied in the field of use of hydroxyprogesterone caproate in the preparation of a drug for treating interstitial pneumonia, and can solve the problem of lack of treatment means, glucocorticoid treatment resistance and side effects , the inability to obtain the effect of glucocorticoid therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1 Establishment of rat model
[0151] SD rats (weight 240-280 g) were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. (animal license number: SCXK (Beijing) 2016-0006; animal certificate number: NO.110011201110788686).
[0152] The quarantine and acclimation period lasted for 1 week, after which they were divided into control group and interstitial pneumonia model group according to body weight. On day 0 of the study, rats in the model group were given a single dose of 5 mg / kg bleomycin by a single intratracheal injection of bleomycin. The rats in the control group were given a single instillation of normal saline. Immediately after completion, the animals were placed upright and rotated to facilitate even distribution of the drug in the lungs. Lie down in a warm environment and wake up naturally. Animals ate and drank freely after they were awake.
Embodiment 2
[0153] Example 2 Experimental grouping
[0154]
[0155]
Embodiment 3
[0156] Example 3 Detected indicators
[0157] General observations and inspections:
[0158] Weight, physical signs, coat status, behavioral activities, presence or absence of nervous reactions such as tremors and spasms, presence or absence of abnormal breath sounds and abnormal gait movements, response to sound shock, stool shape and color, etc. During administration, observe whether there is dirt and excrement in the lower abdomen and anus of the animals, the reactivity when caught, the condition around the eyes, nose and mouth, and the condition of the skin (with or without color change, trauma, tumor, etc.).
[0159] Bronchoalveolar lavage fluid collection
[0160] After the last administration, rats in each group were fasted for 12 hours, and then anesthetized by intraperitoneal injection of 100 mg / L chloral hydrate (3.5 mL / kg). Inject 2 ml of PBS into the lung tissue with a tracheal syringe, gently aspirate it, centrifuge at 2500 r / min for 10 min, aspirate the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com