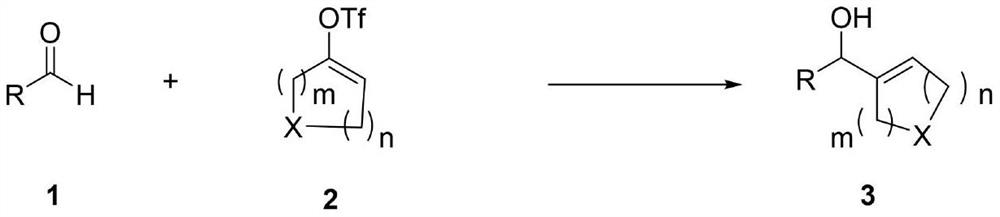Preparation method of allyl alcohol compound
A compound and allyl alcohol technology, applied in the field of preparing allyl alcohol compounds, can solve problems such as insufficient research, and achieve the effects of easy availability of raw materials, simple method operation and little impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Under nitrogen protection, to NiBr 2 (0.01mmol, 0.02eq), L 3 (0.011mmol, 0.022eq) and anhydrous CH 3 CrCl was added to CN(0.1M) solution 2 (0.1 mmol, 0.2 eq), HE (1.5 mmol, 3 eq), 1a (0.5 mmol, 1 eq) and 2a (1 mmol, 2 eq). The reaction mixture was stirred under 10W 400 nm LED irradiation for 48 hours at room temperature. After the reaction was completed, the solvent was evaporated in vacuo, and the crude product was purified by flash column chromatography (petroleum ether / ethyl acetate=5:1) to obtain compound 3a: a colorless oil in 76% yield (83 mg).
[0033] 1 H NMR (400MHz, CDCl 3 )δ7.29-7.25(m,2H),7.20-7.16(m,3H),5.67(dd,J=2.8,1.5Hz,1H),4.15(d,J=2.8Hz,2H),4.03(t , J=6.6Hz, 1H), 3.91-3.65(m, 2H), 2.81-2.51(m, 2H), 2.21-2.13(m, 1H), 2.11-2.02(m, 1H), 1.93-1.80(m , 3H). 13 C NMR (101MHz, CDCl 3 )δ141.75,137.80,128.37,128.35,125.82,121.43,74.48,65.15,64.17,36.18,31.81,24.02.HRMS:Calc’d for C 14 H 17 O,[M-OH] + 201.1274; found 201.1271.
Embodiment 2
[0035] With reference to the reaction conditions in Example 1, by changing different reaction substrates, the results are as follows:
[0036] Colorless oil in 61% yield (55 mg). 1 H NMR (400MHz, CDCl 3 )δ5.63(t,J=2.3Hz,1H),4.14(q,J=2.7Hz,2H),3.84(dt,J=10.6,5.1Hz,1H),3.76-3.71(m,2H), 2.25-2.17(m,1H),2.15-1.94(m,2H),1.81(dtd,J=12.2,7.4,4.6Hz,1H),1.72-1.46(m,6H),1.40(dq,J=13.0 ,8.1,7.6Hz,1H),1.32-1.08(m,1H). 13 C NMR (101MHz, CDCl 3 )δ137.75,122.22,80.31,65.14,64.31,43.11,29.24,29.17,25.65,25.54,23.87.HRMS:Calc’d for C 11 H 17 O,[M-OH] + 165.1274; found165.1272.
[0037] Colorless oil in 63% yield (62 mg). 1 H NMR (400MHz, CDCl 3)δ5.61(t,J=2.3Hz,1H),4.15(q,J=2.7Hz,2H),3.95-3.53(m,3H),2.18(dtt,J=16.7,4.4,2.2Hz,1H ),2.08-1.85(m,2H),1.84-1.58(m,4H),1.58-1.36(m,2H),1.34-1.05(m,3H),1.01-0.88(m,2H). 13 CNMR (101MHz, CDCl 3 )δ136.97,122.48,80.35,76.68,65.20,64.28,40.47,29.58,28.57,26.40,26.13,25.93,23.99.HRMS:Calc’d for C 12 H 19 O,[M-OH] + 179.1430; found179.1427. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com