Alkali-resistant protein A variants and uses thereof
A protein and alkali-resistant technology, applied in the field of protein A variants, can solve problems such as limited application, low mechanical strength, and inability to tolerate high-concentration NaOH cleaning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Directional optimization method of alkali-resistant protein A variant
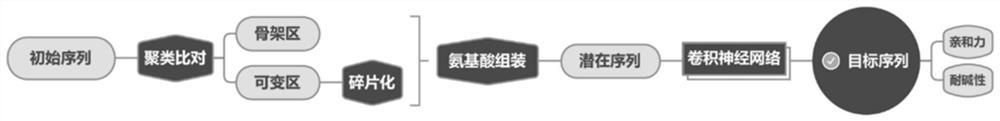
[0043] Aiming at the protein function optimization goals of improving alkali resistance and improving the binding ability with IgG, the present invention obtains the protein A variant with targeted optimization through bioinformatics and convolutional neural network.
[0044] The specific technical solution of the method may include the following steps (see figure 2 ):
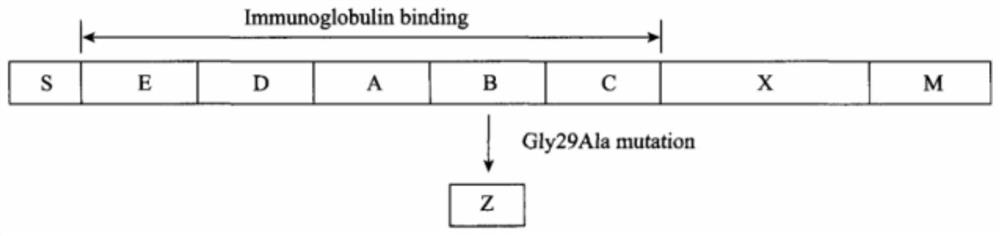
[0045] Step 1: Take the E, D, C, B and A domains and Z domain sequences of protein A as the initial sequence for directional optimization, and obtain the crystal structure of the corresponding sequence from the RCSB-PDB database, where the A domain The structure was obtained by homology modeling method.
[0046] Step 2: Cross-align the initial sequences and perform structural overlay clustering analysis on amino acid residues.
[0047] Step 3: According to the results of sequence identity and amino acid residue structu...
Embodiment 2
[0080] Example 2 Expression and purification of candidate protein A variants
[0081] The above-mentioned ten amino acid sequences from T_SEQAA-1 to T_SEQAA-10 and the Z domain sequence were optimized by using the dominant codons of Escherichia coli to obtain the following corresponding DNA coding sequences.
[0082] T-SEQDNA-1 (SEQ ID NO: 11)
[0083] ATTGATAACAAATTTAACGAAGAACAGCAGGCGGCGTTTTATGAAGTGCTGCATATGCCGAACCTGAACGCGGAACAGCGTAACGGCTTTATTCAGAGCCTGAAAGATGATCCGAGCCAGAGCACCAACCTGCTGGCGGAAGCGCAGAAACTGAACGAAGCGCAGGCGCCGAAA
[0084] T_SEQDNA-2 (SEQ ID NO: 12)
[0085] CAGGATAACCAGTTTAACAAAGAACAGCAGAACGCGTTTTATCAGATTCTGCATCTGCCGAACCTGAACGCGGAACAGCGTAACGCGTTTATTCAGAGCCTGCGTCATGATCCGAGCCAGAGCCTGAACCTGCTGGGCGAAGCGCAGAAACTGAACGATAGCCAGGCGCCGAAA
[0086] T_SEQDNA-3 (SEQ ID NO: 13)
[0087] GCGCAGAACAAATTTGATAAAGAACAGCAGAACGCGTTTTATCAGATTCTGCATATGCCGAACCTGACCGCGGATCAGCGTAACGGCTTTATTCAGAGCCTGAAAGATGATCCGAGCCAGAGCGCGAACGTGCTGGCGGAAGCGCAGAAACTGAACGATGCGCAGGCGCCGAAA
[0088] T_SEQDNA...
Embodiment 3
[0109] Example 3 Conjugation of candidate protein A variants and microspheres
[0110] In this example, agarose microspheres with high particle size uniformity and good mechanical strength were used as scaffolds to couple candidate protein A variants (1#, 3# and 10#) with similar affinity to the Z domain.
[0111] The brief coupling procedure is as follows.
[0112] Step 1: Measure 5mL of epoxy-activated microspheres and wash twice with 10mL of 0.1M PB, pH 8.6 buffer;
[0113] Step 2: Add 3 mg of candidate protein A variant per ml of filler;
[0114] Step 3: React at 30℃±1℃ for 1 hour;
[0115]Step 4: Wash the gel with 20ml of water each time, wash twice in total, collect the washing liquid to measure the protein concentration (OD280), and calculate the protein A variant content on the coupling;
[0116] Step 5: Wash the gel with 20ml of water, and then wash it 5 times;
[0117] Step 6: Wash twice with 5ml 20% ethanol;
[0118] Step 7: 3mL of 20% ethanol, stored at 2-8°C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com