Construction method and application of non-alcoholic steatohepatitis mouse model based on PEDF/ApoE double-gene knockout
A steatohepatitis, non-alcoholic technology, applied in the medical field, can solve the problems of evaluation, inability to simultaneously simulate the pathological characteristics and disadvantages of NASH pathogenesis, and achieve the effect of simple and easy method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Animal body weight, blood lipid content, serum insulin content and blood glucose were measured.
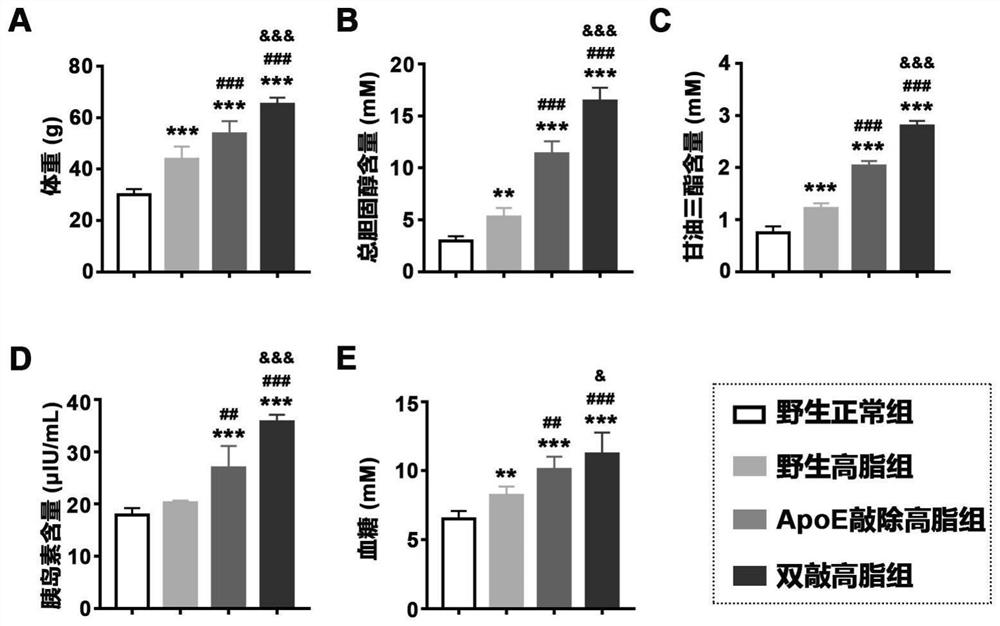
[0029] At the 24th week of induction, the animals in the above four groups were fasted for 12-16 hours, weighed, and blood glucose was measured by a blood glucose meter, and the mice were dissected to collect serum samples, and commercial kits were used to detect serum total cholesterol, triglyceride and insulin levels. , the result is figure 1 shown, wherein A: body weight; B: serum total cholesterol content; C: serum total triglyceride content; D: serum insulin content; F: blood glucose; **, p<0.01, vs wild normal group; ***, p<0.001, vs. wild-type normal group; ##, p<0.01, vs. wild-type high-fat group; ###, p<0.001, vs. wild-type high-fat group; &, p<0.05, vs. ApoE knockout high-fat group; &&& , p<0.001, vs ApoE knockout high-fat group.
[0030] Nonalcoholic steatohepatitis is often associated with obesity, dyslipidemia, insulin resistance, and hyperglycemia, figure 1...
Embodiment 2
[0032] The above-mentioned four groups of animals were evaluated for liver weight, liver-to-body ratio, liver function enzyme levels, liver steatosis, liver inflammation and liver fibrosis at 24 weeks of induction.
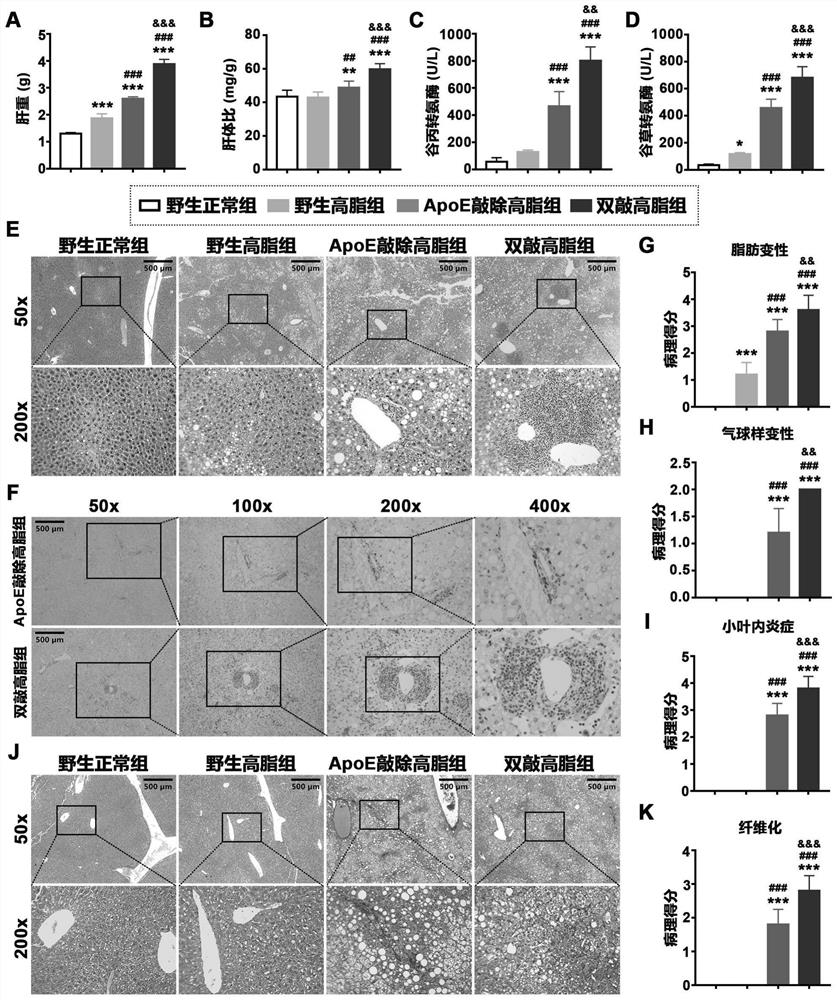
[0033] At the 24th week of induction, animals were fasted for 12-16 hours and then sacrificed by anesthesia. Serum and tissue samples were collected, and liver tissue was weighed; serum alanine aminotransferase and aspartate aminotransferase levels were detected using commercial kits; Sections were stained with HE and Masson to evaluate liver lesions. The results are as follows figure 2As shown, where A: liver weight; B: liver body ratio; C: serum alanine aminotransferase level; D: serum aspartate aminotransferase level; E: HE staining; F: CD45 immunohistochemical staining; G: liver steatosis score; H: liver ballooning lesion score; I: liver lobular inflammation score; J: Masson staining; K: liver fibrosis score; *, p<0.05, vs wild-type normal group; **, p<0.01, ...
Embodiment 3
[0038] The number of liver tumors in the above four groups of animals was counted and the tumors were identified when the animals were induced for 24 weeks.
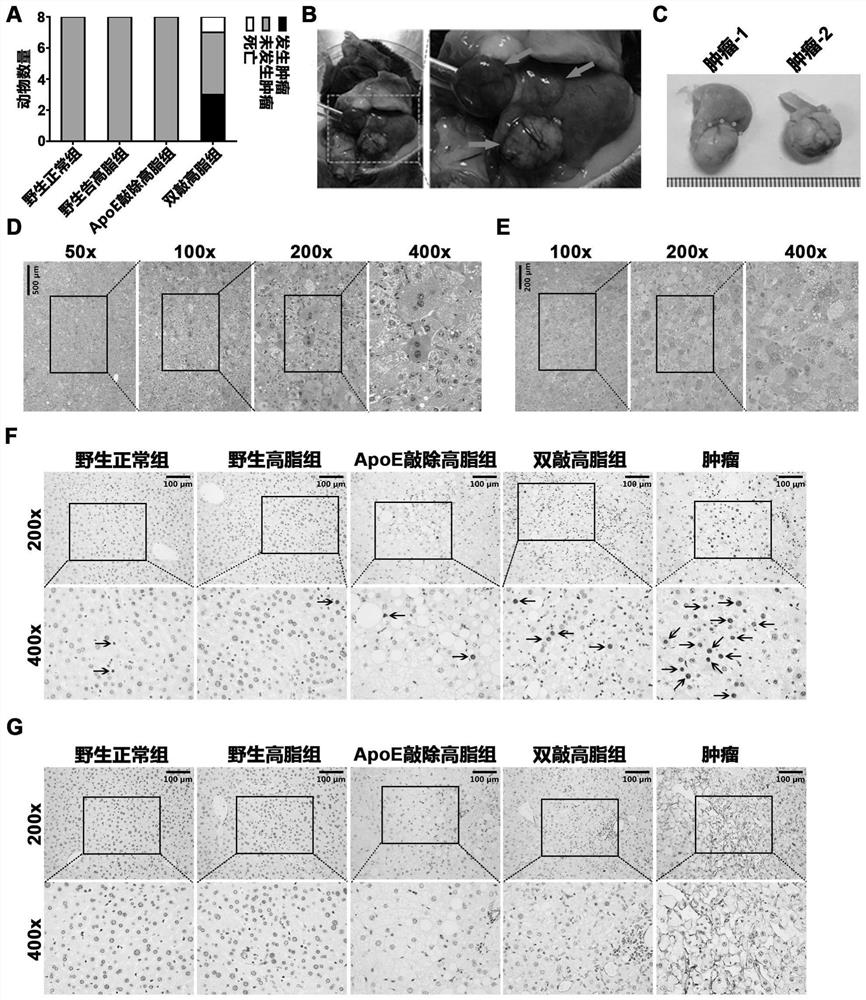
[0039] At the 24th week of induction, animals were fasted for 12 hours and then sacrificed by anesthesia. Serum and tissue samples were collected, and the number of mice that died and developed liver tumors in each group was counted. Paraffin sections were stained with HE and immunohistochemical staining of AFP, PCNA, and CD31 to further clarify tumor lesions. The results are as follows: image 3 As shown in the figure, where A: statistics of tumor occurrence; B: PET / CT results of tumor mice; C: general picture of tumor tissue (after fixation); D: HE staining of tumor tissue; E: AFP staining of tumor tissue; F: tumor tissue PCNA immunohistochemistry; G: tumor tissue CD31 immunohistochemistry.
[0040] Some patients with nonalcoholic steatohepatitis will develop into hepatocellular carcinoma, showing the characteristics ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com