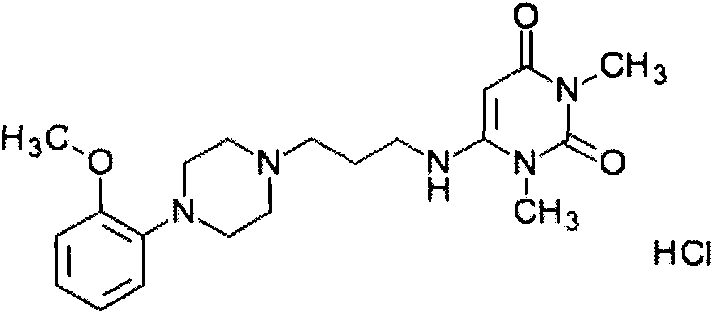
Preparation method of urapidil hydrochloride injection
A technology for urapidil hydrochloride and injection, which is applied in the preparation of pharmaceutical preparations and the field of preparation of urapidil hydrochloride injection, can solve problems such as residues, complex preparation methods, long process time, etc., and achieves stable product quality and is suitable for commercial use. The effect of chemical production and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
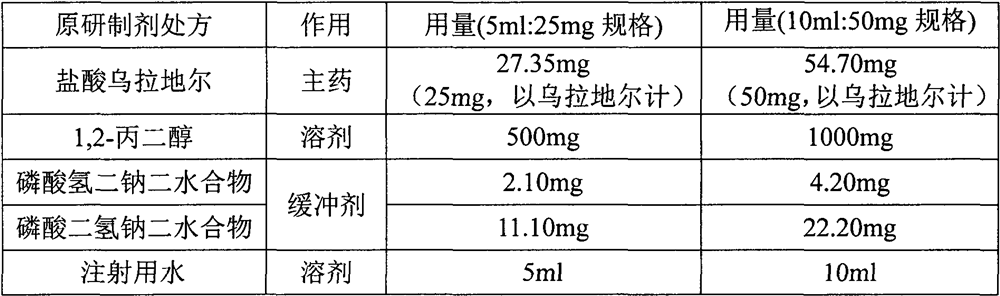
[0059] Preparation:
[0060] (1) Weigh about 4000 g of water for injection below 30°C, and continuously fill with nitrogen;
[0061] (2) under continuous nitrogen filling and stirring, add the 1,2-propanediol of the recipe quantity, and stir to dissolve;
[0062] (3) under continuous nitrogen filling and stirring, add disodium hydrogen phosphate dihydrate and sodium dihydrogen phosphate dihydrate, and stir to dissolve;
[0063] (4) Under continuous nitrogen filling and stirring, add urapidil hydrochloride, stir to dissolve, and measure the pH value (5.5~6.5);
[0064] (5) Add water for injection (below 30°C) to 5000mL;
[0065] (6) Filtration through a 0.22 μm PES filter, filling after filtration, and sterilization at 121° C. for 15 minutes to obtain urapidil hydrochloride injection containing 5.47 mg (5 mg, in urapidil) per 1 ml of urapidil hydrochloride.
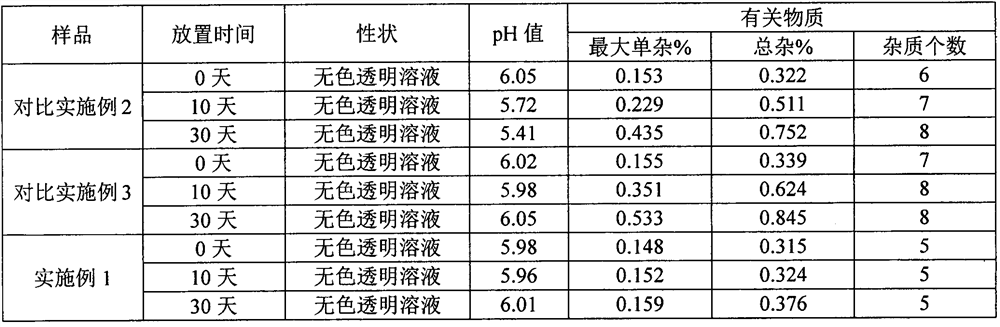
[0066] According to the general rule 1143 of Chinese Pharmacopoeia in 2020, the bacterial endotoxin of Comparative Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com